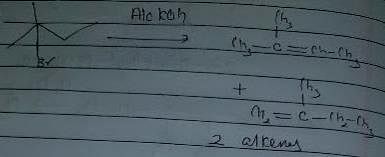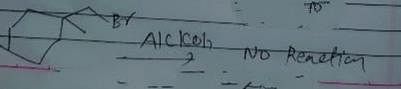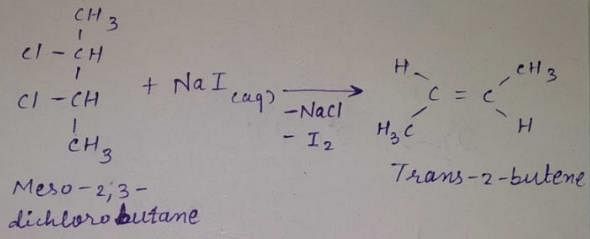Test: Alkene Preparation - NEET MCQ
20 Questions MCQ Test Chemistry Class 11 - Test: Alkene Preparation
Direction (Q. Nos. 1 - 8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
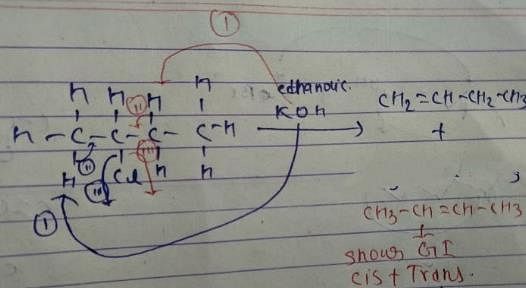
Q. How many different alkenes are formed when 2 -chlorobutane is treated with ethanolic solution of KOH?
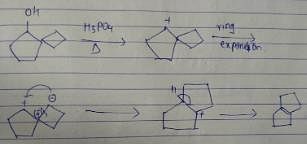
What is the major dehydration product in the following reaction ?
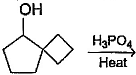
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
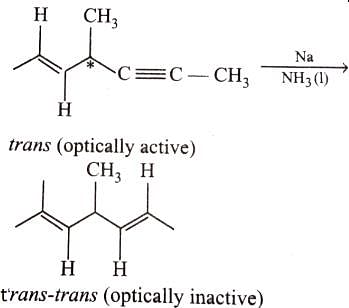
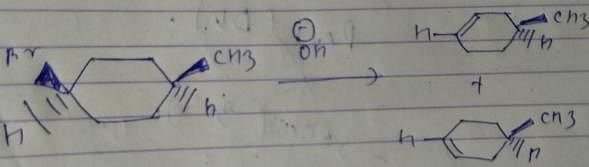
Which of the following compounds will lose optical activity after the reaction ?
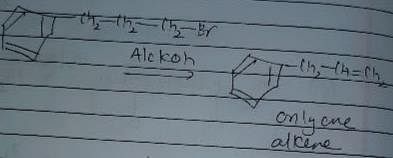
In which of the following reactions , only single is omer of alkene is formed ?
Consider the following reaction .
The correct statement concerning product of the above reaction is
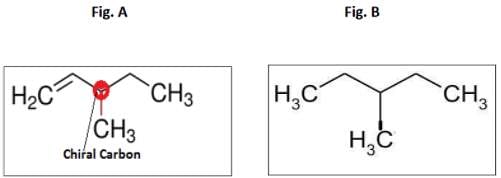
An optically active hydrocarbon X has molecular formula C6H12. X on catalytic hydrogenation gives optically inactive C6H14. X could be
What is the major product of the reaction given below ?
meso -2 ,3 -dichiorobutane + Nal (aq) →
Direction (Q. Nos. 9 -12) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q. Which of the following reactions results in the formation of an alkene?
Which of the following reaction(s) produces propene as one of the important organic product?
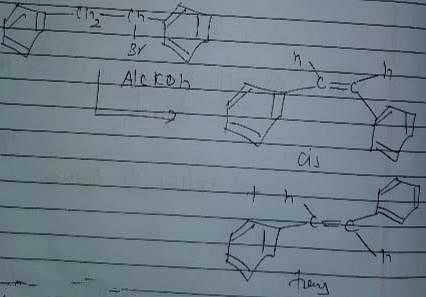
Consider the following reaction.
The correct statement concerning product alkene(s) is/are
Identify the addition reaction which is not undergone by the alkenes
Direction (Q, Nos. 13 - 16) This section is based on Statement I and Statement II. Select the correct answer from the codes given below.
Q.
Statement I : 2-butyne on reduction with Pd/CaCO3 gives c/s-2-butene.
Statement II : Hydrogenation proceed through adsorption mechanism.
Statement I : 2, 3-dibromo butane with Zn-dust gives frans-2-butene as major product.
Statement II : frans-2-butene is more stable than c/s-2-butene.
Statement I : 2-bromobutane with (CH3)3COK in tertiary butanol gives 1 -butene as major product.
Statement II : Very strong base (CH3)3COK in tertiary butanol brings about E-2 elimination reaction.
Statement I : Boiling point of an alkene is slightly greater than that of its hydrogenated alkane.
Statement II : Alkenes have stronger van der Waals’ force of attraction than alkanes.
Direction (Q. Nos. 17 - 20) This section contains 4 questions. When worked o u t wili result in an integer from 0 to 9 (both inclusive).
Q. If 3-bromo-4-methyl hexane is treated with ethanolic KOH solution, how many different alkenes would be formed?
If 3-methyl-2-pentanol is heated with concentrated H2SO4 , in principle how many different alkenes result?
How many different alkyne isomers upon hydrogenation in the presence of CaCO3 / Pd gives 4-methyl hexene which is simultaneously c/s at double bond?
How many different heptenes result by partial hydrogenation of all possible, unbranched heptynes?
|
129 videos|244 docs|88 tests
|