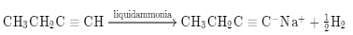Test: JEE Previous Year Questions- Hydrocarbons - JEE MCQ
24 Questions MCQ Test Chapter-wise Tests for JEE Main & Advanced - Test: JEE Previous Year Questions- Hydrocarbons
The compound
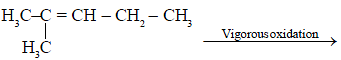 product,
product,
here product is
[AIEEE-2002]
Reaction
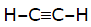 + HOCl → product,
+ HOCl → product,
here product will be -
[AIEEE-2002]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Acetylene does not react with -
[AIEEE-2002]
On mixing a certain alkane with chlorine and irradiating it with ultraviolet light, it forms only one monochloroalkane. This alkane could be -
[AIEEE-2003]
Butene-1 may be converted to butane by reaction with –
[AIEEE-2003]
During dehydration of alcohols to alkenes by heating with conc. H2SO4 the initiation step is-
[AIEEE-2003]
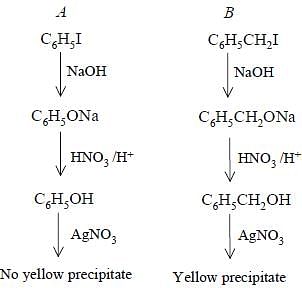
Bottles containing C6H5I and C6H5CH2I lost their original labels. They were labelled A and B for testing. A and B were separately taken in test tubes and boiled with NaOH solution. The end solution in each tube was made acidic with dilute HNO3 and then some AgNO3 solution was added. Substance B gave a yellow precipitate.
Which one of the following statements is true for this experiment ?
[AIEEE-2003]
Which one of the following has the minimum boiling point?
[AIEEE-2004]
Amongst the following compounds, the optically active alkane having lowest molecular mass is -
[AIEEE-2004]
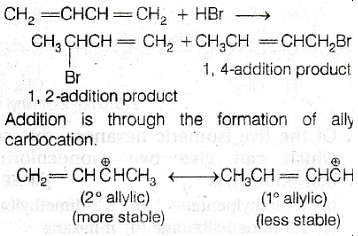
Reaction of one molecule of HBr with one molecule of 1,3–butadiene at 40ºC given predominantly
[AIEEE-2005]
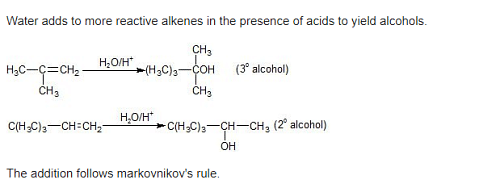
Acid catalyzed hydration of alkenes except ethene leads to the formation of –
[AIEEE-2005]
Alkyl halides react with dialkyl copper reagents to give
[AIEEE-2005]
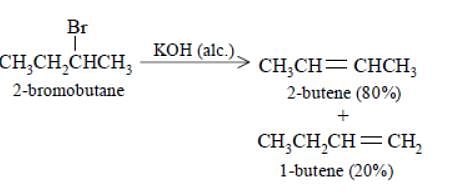
Elimination of bromine from 2–bromobutane results in the formation of –
[AIEEE-2005]

The alkene formed as a major product in the above elimination reaction is
[AIEEE 2006]
Fluorobenzene (C6H5F) can be synthesized in the laboratory -
[AIEEE 2006]
Phenyl magnesium bromide reacts with methanol to give -
[AIEEE 2006]
Which of the following reactions will yield 2, 2 - dibromopropane?
[AIEEE 2007]
In the following sequence of reactions, the alkene affords the compound ‘B’
The compound B is
[AIEEE 2008]
The treatment of CH3MgX with produces
[AIEEE 2008]
The hydrocarbon which can react with sodium in liquid ammonia is -
[AIEEE 2008]
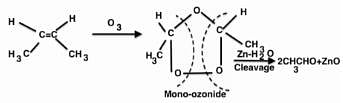
Ozonolysis of an organic compounds gives formaldehyde as one of the products. This confirms the presence of :
[AIEEE 2011]
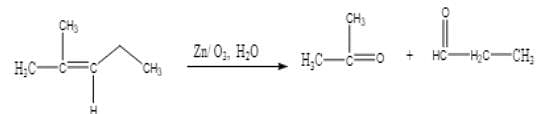
Ozonolysis of an organic compound ‘A’ produces acetone and propionaldehyde in equimolar mixture. Identify ‘A’ from the following compounds :
[AIEEE 2011]
2-Hexyne gives trans -2- Hexene on treatment with -
[AIEEE 2012]
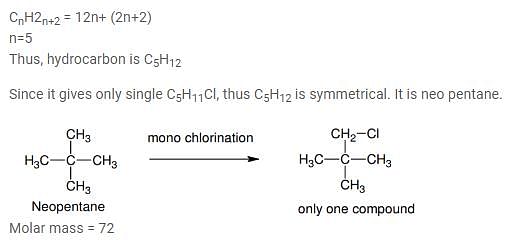
Which branched chain isomer of the hydrocarbon with molecular mass 72u gives only one isomer of mono substituted alkyl halide ?
[AIEEE 2012]
|
446 docs|930 tests
|
|
446 docs|930 tests
|