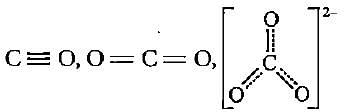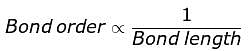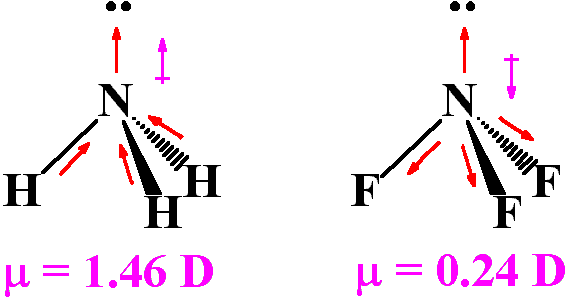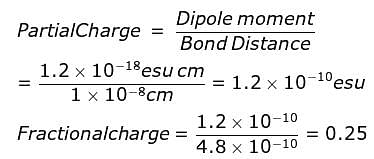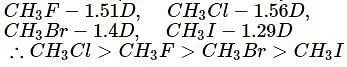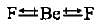Test: Bond Parameters (NCERT) - NEET MCQ
15 Questions MCQ Test NCERTs at Fingertips: Textbooks, Tests & Solutions - Test: Bond Parameters (NCERT)
The correct order of decreasing bond lengths of CO, CO2 and C032- is
The correct sequence of bond length in single bond,double bond and triple bond of C is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Arrange the following in increasing order of covalent character - NaCl, MgCl2, AICI3
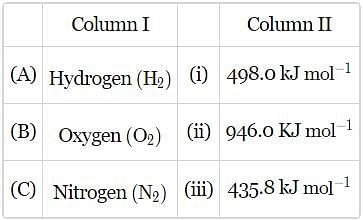
Match the bond enthalpies given in column II with the molecules given in column I and mark the appropriate choice.
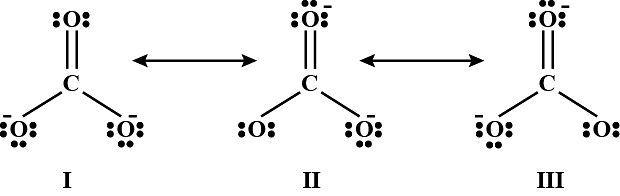
The given structures I, II and III of carbonate ion represent
Which of the following molecules does not show any resonating structures?
The canonical or resonating structures of a molecule required to describe the structure of a molecule follow which of the following rules?
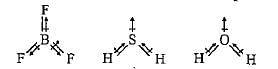
Arrange the following in order of increasing dipole moment: H2O, H2S,BF3
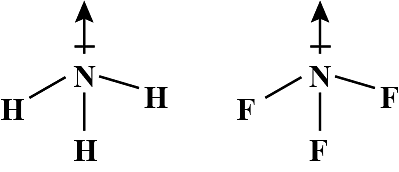
Although F is more electronegative than H, the resultant dipole moment of NH3 is much more than that of NF3. It can be explained as
In a diatomic molecule the bond distance is 1X10-8 cm. Its dipole moment is 1.2 D. What is the fractional electronic charge on each atom?
Which of the following are arranged in the decreasing order of dipole moment?
In water molecule, the two O—H bonds are oriented at an angle of 104.5°. In BF3, the tliree B—F bonds are oriented at an angle of 120°. In BeF2, the two Be—F bonds are oriented at an angle of 180°. Which of the following will have highest dipole moment?
What is the correct dipole moment of NH3 and NF3 respectively?
|
257 docs|234 tests
|
|
257 docs|234 tests
|