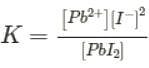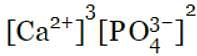Test: Solubility Product - NEET MCQ
15 Questions MCQ Test Topic-wise MCQ Tests for NEET - Test: Solubility Product
. What is the molar solubility (s) of Ba3(PO4)2 in terms of Ksp?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The solubility product expression for tin(II) hydroxide, Sn(OH)2, is
The [Ag+(aq)] = 10-5 in a solution .The [Cl–(aq)] to precipitate AgCl having Ksp of 1.8×10-10 M2 is — M
The molar solubility of PbBr2 is 2.17 x 10-3 M at a certain temperature. Calculate Kspfor PbBr2
What is the correct equilibrium expression (Ksp) for the reaction below:
Ca3(PO4)2(s)  3Ca2+(aq) + 2PO43-(aq)
3Ca2+(aq) + 2PO43-(aq)
When in a saturated solution of NaCl, HCl is passed, pure precipitate of NaCl is formed. This is due to the fact:
The solubility product expression for silver(I) sulphide, using x to represent the molar concentration of silver(I) and y to represent the molar concentration of sulphide, is formulated as:
Consider the following solubility data for various chromates at 25°C.
Salt Ksp
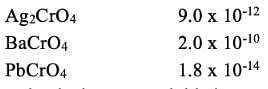
the chromate that is most suitable is?
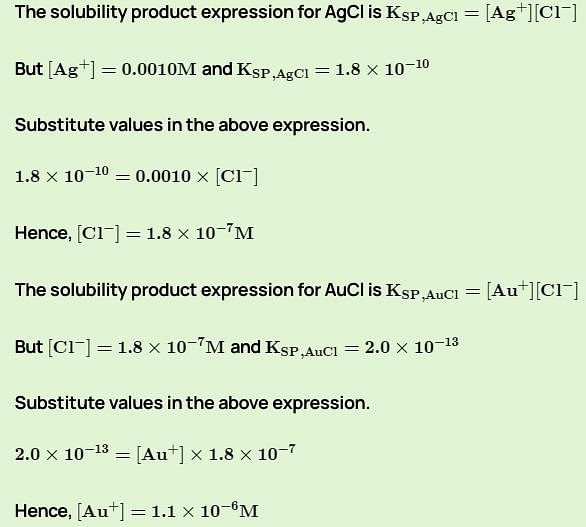
A solution is 0.0010 M in both Ag+ and Au+. Some solid NaCl is added slowly until the solid AgCl just begins to precipitate. What is the concentration of Au+ ions at this point? Ksp for AgCl = 1.8 x 10-10 and for AuCl = 2.0 x 10-13.
|
9 docs|1272 tests
|