Test: Hydrogen Bonding - NEET MCQ
17 Questions MCQ Test Chemistry Class 11 - Test: Hydrogen Bonding
Direction (Q. Nos. 1-12) This section contains 12 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Which of the following compounds are soluble in H2O ?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
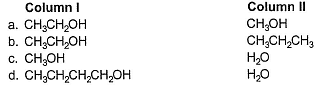
In which of the following boiling point of Column I is not higher than that of Column II?
In dimer formation of which O-atom(s) is/are involved through H-bonding?
In the following compounds, select the points of interm olecular H-bonding (linear),
In the following alcohol, which — OH group is involved to the maximum extent in H-bonding?
Among the following sets, highest boiling points are of the species.
I. HF, HCI, HBr, HI
II. H2O, H2S, H2Se, H2Te
III. NH3,PH3, AsH3,SbH3
What is the dominant intermolecular force or bond that must be overcome in converting liquid CH3CH2OH to vapours?
Number of water molecules directly attached to one water molecule is
Consider the following compounds
I. HCI
II. HF
III. CH3COOH
IV. CH4
V. CH3OH
VI. CH3COO-
Q.
H-bonding is not present in
Direction (Q. Nos. 13-16) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q.
In which of the following I is more volatile than II?
Hydrogen bonding plays a central role in the following phenomenon
[JEE Advanced 2014]
Which of the following molecules are stabilised by intramolecular H-bonding?
Direction (Q. Nos. 17) Choice the correct combination of elements and column I and coloumn II are given as option (a), (b), (c) and (d), out of which ONE option is correct.
Q. Match the species in Column I with rank order of their boiling point in Column II. (Species with lowest boiling points is at SN1).
|
129 videos|244 docs|88 tests
|












