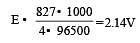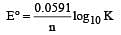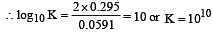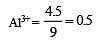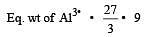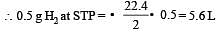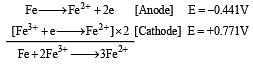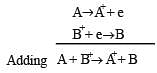31 Year NEET Previous Year Questions: Electrochemistry - 2 - NEET MCQ
30 Questions MCQ Test Chemistry Class 12 - 31 Year NEET Previous Year Questions: Electrochemistry - 2
On electrolysis of dilute sulphuric acid using platinum electrodes, the product obtained at the anode will be [1992]
Which of the following is an insulator ? [1992]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The standard reduction poten tials at 25°C of
Li+/ Li, Ba 2+ / Ba, Na+ / Na
and Mg 2+ / Mg are – 3.03, – 2.73, – 2.71 and – 2.37 volt respectively. Which one of the following is the strongest oxidising agent? [1994]
Li+/ Li, Ba 2+ / Ba, Na+ / Na
and Mg 2+ / Mg are – 3.03, – 2.73, – 2.71 and – 2.37 volt respectively. Which one of the following is the strongest oxidising agent? [1994]
The most durable metal plating on iron to protect against corrosion is [1994]
On heating one end of a piece of a metal, the other end becomes hot because of [1995]
If 0.5 amp current is passed through acidified silver nitrate solution for 100 minutes. The mass of silver deposited on cathode, is (eq.wt.of silver nitrate = 108) [1995]
An electr och emical cell is set up as: Pt; H2 (1atm)|HCl(0.1 M) || CH3COOH (0.1 M)| H2 (1atm); Pt. The e.m.f of this cell will not be zero, because [1995]
On passing a current of 1.0 ampere for 16 min and 5 sec through one litre solution of CuCl2, all copper of the solution was deposited at cathode. The strength of CuCl2 solution was (Molar mass of Cu= 63.5; Faraday constant = 96,500 Cmol–1) [1996]
Equivalent conductances of NaCl, HCl and CH3COONa at infinite dilution are 126.45, 426.16 and 91 ohm–1 cm2 respectively. The equivalent conductance of CH3COOH at infinite dilution would be [1997]
If 0.01 M solution of an electrolyte has a resistance of 40 ohms in a cell having a cell constant of 0.4 cm–1, then its molar conductance in ohm–1 cm2 mol–1 is [1997]
Eº for the cell, 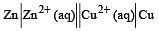
1.10 V at 25ºC. The equilibrium constant for the cell reaction:
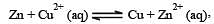
Standard potentials (Eº) for some half-reactions are given below :
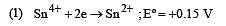
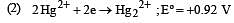
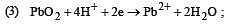 Eº = +1.45 V
Eº = +1.45 V
Based on the above, which one of the following statements is correct ? [1997]
For the cell reaction, [1998] Cu2+ (C1, aq) + Zn(s) = Zn2+ (C2, aq) + Cu(s) of an electrochemical cell, the change in free energy, ΔG, at a given temperature is a function of
Without losing its concentration ZnCl2 solution cannot be kept in contact with [1998]
What is the Eºcell for the reaction
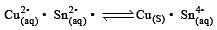
at 25ºC if the equilibrium constant for the reaction is 1 × 106? [1999]
Th e ionic conductan ce of Ba2+ an d Cl– are respectively 127 and 76 ohm–1 cm2 at infinite dilution. The equivalent conductance (in ohm–1 cm2) of BaCl2 at infinite dilution will be : [2000]
Cu +aq is unstable in solution and undergoes simultaneous oxidation and reduction according to the reaction : [2000]
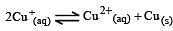
choose correct Eº for above reaction if Eº Cu2+/Cu = 0.34 V and Eº Cu2+/Cu+ = 0.15 V
Specific conductance of a 0.1 N KCl solution at 23ºC is 0.012 ohm–1 cm–1. Resistance of cell containing the solution at same temperature was found to be 55 ohm. The cell constant is [2000]
Standard electrode potentials are : Fe+2/Fe [ Eº = –0.44]; Fe+3/Fe+2 Eº = + 0.77 ; If Fe+2, Fe+3 and Fe blocks are kept together, then [2001]
The most convenient method to protect the bottom of ship made of iron is [2001]
In the silver plating of copper, K[Ag(CN)2] is used instead of AgNO3. The reason is [2002]
Which reaction is not feasible? [2002]
In electrolysis of NaCl when Pt electrode is taken then H2 is liberated at cathode while with Hg cathode it forms sodium amalgam. This is because [2002]
The e.m.f. of a Daniell cell at 298 K is E1.

When the concentration of ZnSO4 is 1.0 M and that of CuSO4 is 0.01 M, the e.m.f. changed to E2.What is the relationship between E1 and E2? [2003]
On the basis of the information available from the reaction
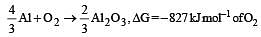
the minimum e.m.f required to carry out an electrolysis of Al2O3 is (F = 96500 C mol–1)
The standard e.m.f. of a galvanic cell involving cell reaction with n = 2 is found to be 0.295 V at 25°C. The equilibrium constant of the reaction would be (Given F = 96500 C mol–1; R = 8.314JK– 1mol–1)[2004]
4.5 g of aluminium (at. mass 27 amu) is deposited at cathode from Al3+ solution by a certain quantity of electric charge. The volume of hydrogen produced at STP from H+ ions in solution by the same quantity of electric charge will be [2005]
If 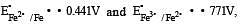 , the standard EMF of the reaction Fe + 2Fe3+ → 3Fe2+ will be [2006]
, the standard EMF of the reaction Fe + 2Fe3+ → 3Fe2+ will be [2006]
A hypoth etical electrochemical cell is shown below 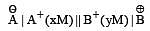 [2006] The emf measured is +0.20 V. The cell reaction is
[2006] The emf measured is +0.20 V. The cell reaction is
The efficiency of a fuel cell is given by [2007]
|
108 videos|286 docs|123 tests
|


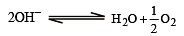

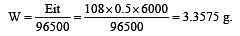
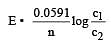
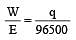
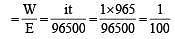
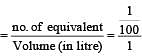
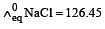
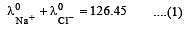
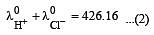
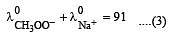
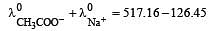
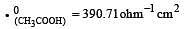
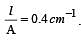
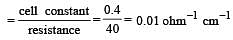
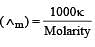
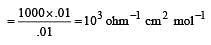
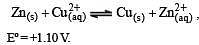
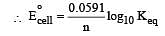
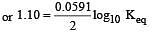
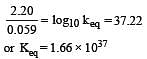
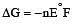
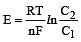
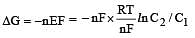
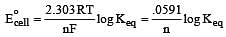
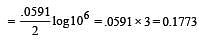
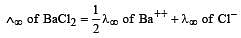
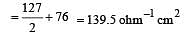
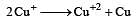
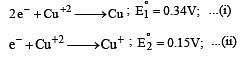
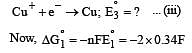
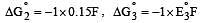
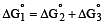
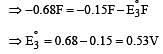
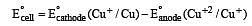
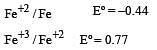


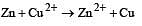
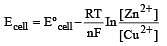
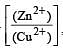 less is the EMF
less is the EMF