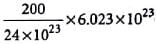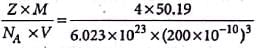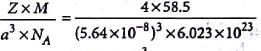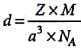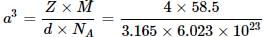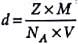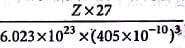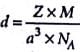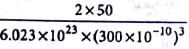NEET Exam > NEET Tests > Chemistry Class 12 > Test: Calculations Involving Unit Cell Dimensions (Old NCERT) - NEET MCQ
Test: Calculations Involving Unit Cell Dimensions (Old NCERT) - NEET MCQ
Test Description
5 Questions MCQ Test Chemistry Class 12 - Test: Calculations Involving Unit Cell Dimensions (Old NCERT)
Test: Calculations Involving Unit Cell Dimensions (Old NCERT) for NEET 2024 is part of Chemistry Class 12 preparation. The Test: Calculations Involving Unit Cell Dimensions (Old NCERT) questions and answers have been
prepared according to the NEET exam syllabus.The Test: Calculations Involving Unit Cell Dimensions (Old NCERT) MCQs are made for NEET 2024 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Calculations Involving Unit Cell Dimensions (Old NCERT) below.
Solutions of Test: Calculations Involving Unit Cell Dimensions (Old NCERT) questions in English are available as part of our Chemistry Class 12 for NEET & Test: Calculations Involving Unit Cell Dimensions (Old NCERT) solutions in
Hindi for Chemistry Class 12 course. Download more important topics, notes, lectures and mock
test series for NEET Exam by signing up for free. Attempt Test: Calculations Involving Unit Cell Dimensions (Old NCERT) | 5 questions in 5 minutes | Mock test for NEET preparation | Free important questions MCQ to study Chemistry Class 12 for NEET Exam | Download free PDF with solutions
Test: Calculations Involving Unit Cell Dimensions (Old NCERT) - Question 1
An element cyrstallises in a structure having a fcc unit cell of and edge 200 pm. Calculate its density if 200 g of this element contains 24 x 1023 atoms.
Detailed Solution for Test: Calculations Involving Unit Cell Dimensions (Old NCERT) - Question 1
Test: Calculations Involving Unit Cell Dimensions (Old NCERT) - Question 2
A unit cell of sodium chloride has four formula units. The edge length of the unit cell is 0.564 nm. What is the density of sodium chloride?
Detailed Solution for Test: Calculations Involving Unit Cell Dimensions (Old NCERT) - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Test: Calculations Involving Unit Cell Dimensions (Old NCERT) - Question 3
The distance between Na+ and CI- ions in NaCl with a density 3.165 g cm-3 is
Detailed Solution for Test: Calculations Involving Unit Cell Dimensions (Old NCERT) - Question 3
Test: Calculations Involving Unit Cell Dimensions (Old NCERT) - Question 4
The unit cell of aluminium is a cube with an edge length of 405 pm. The density of aluminium is 2.70 g cm-3. What is the structure of unit cell of aluminium?
Detailed Solution for Test: Calculations Involving Unit Cell Dimensions (Old NCERT) - Question 4
Test: Calculations Involving Unit Cell Dimensions (Old NCERT) - Question 5
The density of a metal which crystallises in bcc lattice with unit cell edge length 300 pm and molar mass 50 g mol-1 will be
Detailed Solution for Test: Calculations Involving Unit Cell Dimensions (Old NCERT) - Question 5
|
108 videos|286 docs|123 tests
|
Information about Test: Calculations Involving Unit Cell Dimensions (Old NCERT) Page
In this test you can find the Exam questions for Test: Calculations Involving Unit Cell Dimensions (Old NCERT) solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Calculations Involving Unit Cell Dimensions (Old NCERT), EduRev gives you an ample number of Online tests for practice