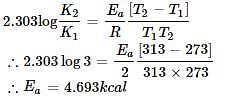Multiple Choice Questions (MCQs): Chemical Kinetics - 2 - NEET MCQ
25 Questions MCQ Test Chemistry Class 12 - Multiple Choice Questions (MCQs): Chemical Kinetics - 2
Study of rate of chemical reaction is known as
Which among the following is an instantaneous reaction?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The rate of reaction can be measured as
Rate of reaction for the combustion of propane is equal to
C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O(g)
What is the order of a reaction which has a rate expression ; Rate =
Molecularity of a reaction
For which of the following reaction the units of rate constant and rate of the reaction are same?
The rate of chemical reaction becomes double for every 10o rise in temperature because of
The rate is independent of the concentration of the reactants in
A reaction whose order is different from the actual due to large excess concentration of one of the reactants is called?
The role of catalyst in a chemical reaction is to change the
Rate of ionic reactions are generally
The metabolism of hormones in human body is an example of
If a reaction proceeds with a uniform rate throughout, the reaction is
Which among the following is an example of photochemistry used in our daily life?
The ionic reactions are generally very fast because
The rate constant of the reaction at temperature 200 K is 10 times less than the rate constant at 400 K. The activation energy of the reaction is
Which among the following is an instantaneous reaction?
The half life of a reaction is halved as the initial concentration of the reactant is doubled. The order of the reaction is
If 75% of a first order reaction was completed in 32 min, then 50% of the reaction was completed in
Milk turns sour at 40°C three times faster than it does at 0°C, this shows that activation energy of souring of milk (in cal) is
In a reaction, A + B → Product, rate is doubled when the concentration of B is doubled, and rate increases by a factor of 8 when the concentrations of both the reactants (Aand B) are doubled, rate law for the reaction can be written as
Thermal decomposition of a compound is of first order. If 50% of a sample of a compound is decomposed in 120 min, the time taken for 90% completion is
In the catalyzed decomposition of benzene diazonium chloride,
Half life period is found to be independent of the initial concentration of the reactant. After 10 min, the volume of N2 gas collected is 10 L and after the reaction is complete, it is 50 L. Hence, the rate constant of the reaction(in min-1) is
Decomposition of H2O2 was studied by titration against KMnO4 solution. It was found that 0.4 mol of H2O2 was reduced to 0.2 mol in 20 min and to 0.1 mol in 40 min and to 0.05 mol after 1 hr, the order of reaction must be
|
108 videos|286 docs|123 tests
|