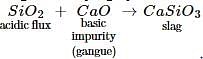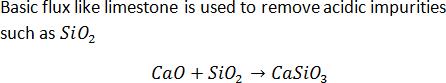Test: Metallurgy Process of Concentration of Ores (Old NCERT) - NEET MCQ
25 Questions MCQ Test NCERT Based Tests for NEET - Test: Metallurgy Process of Concentration of Ores (Old NCERT)
Only One Option Correct Type
This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct
Q.
Flux is often added to remove impurities from an iron ore in a blast furnace. In the reaction slag and flux are
CaO + SiO2 →CaSiO3
The slag and the flux are
Froth flotation process is based on
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which one of the following ores is concentrated by chemical leaching method?
Which of the ore dressing process requires finest size of ore?
Wolframite(FeWO4) impurities present in cassiterite (SnO2) can be separated by the process of
In the metallurgical process, the flux used for removing acidic impurities is
Gravity method of ore concentration depends on
Sulphide ores of metals are usually concentrated by froth floatation process.Which one of the following sulphide ores offers an exception and is concentrated by leaching?
The slag formed in the extraction of iron from haematite in the blast furnace is:
In the froth floatation process for benefaction of the ores, the ore particles float because
One or More than One Options Correct Type
This section contains 5 multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.?
Q.
The ore that is concentrated by froth floatation process is
In froth floatation process the particles of the ore come on the froth because
Purification of bauxite is called
The common impurities found in bauxite are
Which of the following chemicals are involved in froth floatation process?
Comprehension Type
This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d).
Passage
Most of the ores available in nature contain large amount of impurities i.e. gangue. The pretreatment of ores, based on physical properties and without bringing out any major chemical change in the ore is called ore dressing or ore concentration.
Q. Which of the following is related to ore concentration?
Most of the ores available in nature contain large amount of impurities i.e. gangue. The pretreatment of ores, based on physical properties and without bringing out any major chemical change in the ore is called ore dressing or ore concentration.
Q.
In the purification of bauxite by leaching, the chemicals employed in Baeyer’s and Hall’s process respectively are
Matching List Type
Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct.
Q.
Match the Column I with Column II and mark the correct option from the codes given below.
Match the Column I with Column II and mark the correct option from the codes given below.
One Integer Value Correct Type
This section contains 5 questions, when worked out will result in an integer value from 0 to 9 (both inclusive).
Q.
The number of ores concentrated by froth floatation process among cassiterite, galena, zinc blende, chromite, haematite, copper iron pyrites
The number of ores concentrated by leaching or wet process among calamine, tinstone, bauxite, argentite, horn silver, galena, cinnabar, native gold
In the leaching of Ag2S with NaCN the equation is
Ag2S + 4NaCN → 2Na[Ag(CN)2] + Na2S. In this change in oxidation state of silver.
Number of ores concentrated by Levigation (hydraulic washing) among pitche blende, tinstone, chromite, galena, copper pyrites, argentite.
The number of magnetic substances among : tinstone, wolframite, chromite, magnetite, pyrolusite, rutile, magnesite, sphalerite
Statement Type
Direction : This section is based on Statements I and Statements II. Select the correct answer from the codes given below.
Q.
Statement I : Cresol and aniline are froth stabilisers.
Statement II : Collectors enhance the non-wettability of the ore particles.
|
748 tests
|