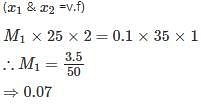P. Bahadur Test: Solutions - NEET MCQ
25 Questions MCQ Test Topic-wise MCQ Tests for NEET - P. Bahadur Test: Solutions
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Molality is preferred over molarity in handling solutions in chemistry laboratory because
Aquatic animals are more comfortable in cold water rather that warm water because
In the depression of freezing point experiment, it is found that
If the temperature of an aqueous solution increases, it will cause
Which among the following is an example of liquid in solid?
Which among the following is an example of a solid solution in which the solute is a gas?
The type of intermolecular interaction present in n-Hexane and n-Octane is
Mathematical expression relating molarity and molality is
The tanks used by divers are filled with air diluted with
Ethylene glycol is added to water as antifreeze. It will
Which of the following solutions of H2SO4 is more concentrated?
An aqueous solution of methanol and water has vapour pressure
A solution showing a large positive deviation from ideal behaviour has
In which unit, the concentration of solution remains independent of temperature
6.3 g oxalic acid is used to make 250 mL aqueous solution .The volume of 0.1N NaOH solution required to neutralize completely 10 mL of this solution is
The depression in freezing point for 1M urea, 1 M glucose and 1 M NaCl are in the ratio of
25ml of a solution of barium hydroxide titration with 0.1molar solution of hydro-chloric acid gave a titre value of 35ml.The molarity of barium hydroxide is
|
9 docs|1272 tests
|