Test: E1 Reactions & Related Reactions - NEET MCQ
15 Questions MCQ Test Topic-wise MCQ Tests for NEET - Test: E1 Reactions & Related Reactions
Only One Option Correct Type
Direction (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q.
The incorrect statement concerning E1 reaction is
The correct statement regarding a unimolecular elimination reaction is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
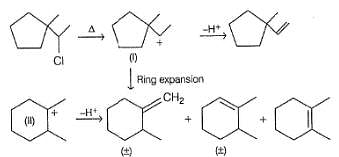
Consider the following reaction,


Q.
The correct statement concerning I and II is


Consider the following elimination reaction,
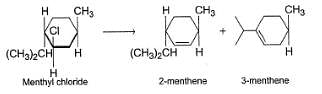
When reaction is carried out with C2H5ONain ethanol, 2-menthene is the major product while 3-menthene is the major product if reaction is substrate is heated in ethanol only. It is due to
The reaction of tertiary butyl chloride in water to yield tertiary butyl alcohol is not appreciably affected by dissolved NaF; in DMSO, however, NaF bring about rapid formation of isobutene. It is because of
When (CH3)3C — Cl is heated in ethanol, both alkene and ether are formed simultaneously by E1 and SN1 reaction. Which of the following will have adverse effect on both of the above reaction?
Consider the following reaction,
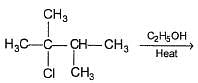
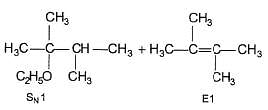
Q.
Increasing temperature increases the proportion of elimination (E1) product because
Which is the least reactive halide in both E1 and SN1 reaction?
Direction (Q. Nos. 9-11) This section contains 3 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Consider the following reaction,
Consider the following elimination reaction,
The correct statement regarding an E1 reaction is/are ?
Comprehension Type
Direction (Q. Nos. 12-14) This section contains a paragraph, describing theory, experiments, data, etc. Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
Consider the following reaction. The tertiary butyl group can be cis or trans to tosylate group. Answer the following questions based on the knowledge of E2 and E1 reaction mechanism.
Q.
The correct statement concerning above elimination reaction is
Consider the following reaction. The tertiary butyl group can be cis or trans to tosylate group. Answer the following questions based on the knowledge of E2 and E1 reaction mechanism.
Q.
When tertiary butyl group and tosylate group are trans, the correct statement is
Consider the following reaction. The tertiary butyl group can be cis or trans to tosylate group. Answer the following questions based on the knowledge of E2 and E1 reaction mechanism.
Q.
If the rate of reaction is independent of NaOEt concentration, it is concluded that
One Integer Value Correct Type
Direction (Q. Nos. 15 and 16) This section contains 2 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Consider the following reaction,
Q.
In principle, how many different alkenes are possible by the above elim ination reaction?
|
9 docs|1272 tests
|