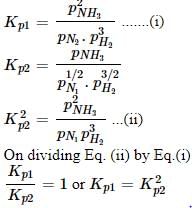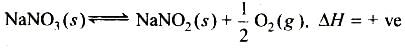Test: Chemical Equilibrium - 1 - Chemistry MCQ
20 Questions MCQ Test Physical Chemistry - Test: Chemical Equilibrium - 1
For the pair of reactions given below,
i) 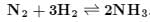
(ii) 1/2N2(g) + 3/2H2(g) ⇔ NH3(g)
If at a particular temperature, Kp1 and Kp2 are the equilibrium constants for reaction (i) and (ii) respectively then,
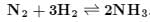
Among the following, the equilibrium which is not affected by an increase in pressure is:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Under the equilibrium condition for the reaction, 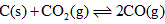 the total pressure is 12 atm. If 50% of CO2 reacts then value of Kp is:
the total pressure is 12 atm. If 50% of CO2 reacts then value of Kp is:
For the reaction, the equilibrium constant Kp changes with:
Pure ammonia is placed in a vessel at a temperature where its dissociation constant (α) is appreciable. At equilibrium,
One mole of N2O4(g) at 300 K is kept in a closed container under one atmosphere. It is heated to 600 K when 20% by mass of N2O4(g) decomposes to NO2(g). The resultant pressure is:
For the reaction:
At a given temperature, the equilibrium amount of CO2(g) can be increased by.
For the chemical reaction,
The amount of X3 Y at equilibrium is affected by
pH of a saturated solution of Ba(OH)2 is 12. The value of solubility product (Ksp) of Ba(OH)2 is
When two reactants, A and B are mixed to give products, C and D, the reaction quotient, (Q) at the initial stages of the reaction:
At constant temperature, the equilibrium (Kp) for the decomposition reaction
is expressed by
where, p = pressure,
X = extent of decomposition. Which one of the following statement is true:
Consider the following equilibrium in a closed container
At a fixed temperature, the volume of the reaction container is halved. For this change, which of the following statements hold true regarding the equilibrium constant (Kp) and degree of dissociation (α)?
Which is correct statement if N2 is added at equilibrium condition?
N2 + 3H2 ⇌ 2NH3
The relationship between the equilibrium constant K1 for the reaction.
CO(g) +1/2O2 (g) CO2(g)
And the equilibrium constant K2 for the reaction:
For the reaction H2 + I2 ⇋ 2HI the equilibrium constant (K) is
For the gas phase reaction.
Carried out in a vessel, the equilibrium concentration of C2H4 can be increased by
(I) Increasing the temperature
(II) Decreasing the pressure
(III) Removing some H2
(IV) Adding some C2H6
When NaNO3 is heated in a closed vessel, oxygen is liberated and NaNO2 is left behind. At equilibrium.
The equilibrium is attained at 250C in a closed container and an inert gas, helium is introduced. Which of the following statements are correct?
For the reaction,
The forward reaction at constant temperature is favoured by
(I) Introducing an inert gas at constant volume
(II) Introducing chlorine gas at constant volume
(III) Introducing an inert gas at constant pressure
(IV) Increasing the volume of the container
(V) Introducing PCl5 at constant volume
For the reaction, the equilibrium constant Kp changes with:
|
83 videos|142 docs|67 tests
|