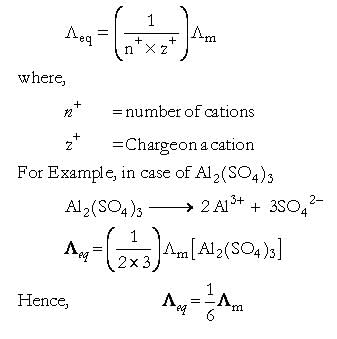Test: Conductance - 2 - Chemistry MCQ
30 Questions MCQ Test Physical Chemistry - Test: Conductance - 2
The correct expressions between conc. mobility and molar conductivity are:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The correct expressions for dissociation constant of weak acid are:
The correct expressions which explain the Debye-Huckel-Onsager Theory for strong electrolytes are
The correct order of molar conductivities at infinite dilution are:
Which of the following will have same value of molar conductivity and equivalent conductivity:
If = 73.5Ω-1cm2 mol-1
= 189Ω-1cm2 mol-1 and
= 160Ω-1cm2 mol-1 then:
Choose the correct order of molar ionic conductivities of the following ions.
The equivalent conductance of 0.01 M solution of K2SO4 whose specific conductance is 1.26 × 10–3 Ω-1 cm-1 are ________ Ω-1cm2 eq-1
The conductivity of 0.1 M NaOH solution is 0.0221 Ω-1 cm-1. When an equal volume of 0.1 M HCl is added, the conductivity decreases to 0.0056 Ω-1 cm-1. A further addit ion HCl solution, the volume of which of which is equal to that of the first portion added, the conductivity increases to 0.017 Ω-1 cm-1.
The conductivity of 0.1 M NaOH solution is 0.0221 Ω-1 cm-1. When an equal volume of 0.1 M HCl is added, the conductivity decreases to 0.0056 Ω-1 cm-1. A further addit ion HCl solution, the volume of which of which is equal to that of the first portion added, the conductivity increases to 0.017 Ω-1 cm-1. ________, _____ and _______ Ω-1 cm-1 respectively:
The specific conductance of a saturated solution of AgCl at 25ºC after subtracting the specific conductance of water is 2.28 × 10–4 Sm–1. The molar ionic conductance of Ag+ and Cl– ions are 73.3 × 10–4 and 65 × 10–4 Sm2 mol–1. The solubility of AgCl at 25ºC is _______ g/dm3.
The ionic conductivities at infinite dilution of Ox2–, K+ and Na+ are 148.2, 50.1 and 73.5 Ω-1 cm2 mol-1 respectively. The equivalent conductivities at infinite dilution of the salt KOOC. COONa is _______ Ω-1 cm2 mol-1
The molar conductivity of NH4Cl at infinite dilution is 149.7 Ω-1 cm2 mol-1 and the ionic conductivities of OH- and Cl- ions are 198 and 76.3 Ω-1 cm2 mol-1 respectively. The molar conductivity of NH4OH at the dilution is _______ Ω-1 cm2 mol-1:
A conductivity cell whose cell constant is 2cm–1 is filled with 0.1 M acetic acid solution. Its resistance is found to be 3765 Ω. The degree of dissociation of acetic acid are ______ . Given that and
The conductivity of a saturated solution of silver okalate is 4.5 × 10–5 Ω-1 cm-1. If its Ksp = 1.35 × 10-11 M3, the molar conductivity of the saturated solution would be _______ Ω-1 cm2 mol-1.
The molar conductivity of 0.05 M of solution of an electrolyte is 200 Ω-1 cm2 mol-1. The resistance offered by a conductivity cell with cell constant (1/3 cm-1) would be _______Ω.
A conductivity cell whose cell constant is 3 cm–1 is filled with 0.1 M solution of weak acids. Its resistance is found to be 3000 Ω. the degree of dissociation of weak acid is _______.
|
83 videos|142 docs|67 tests
|