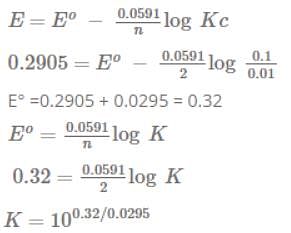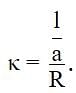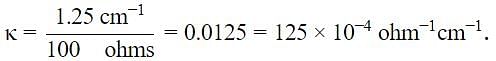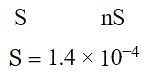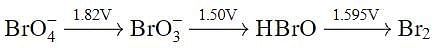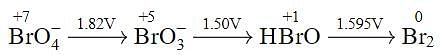Test: Electrochemistry - 1 - NEET MCQ
20 Questions MCQ Test Chemistry Class 12 - Test: Electrochemistry - 1
The standard reduction potential at 298 K for the following half cells are given:
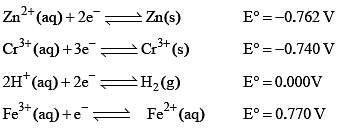
Which is the strongest reducing agent:
Which of the following conditions are satisfied when the cell reaction in the electrochemical cell is spontaneous?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
What is the observation when the opposing external applied potential to an electrochemical cell is greater than the cell’s potential?
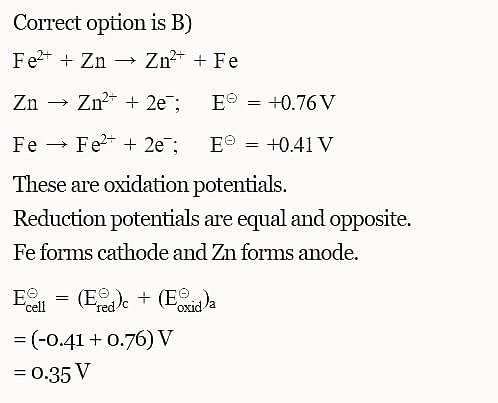
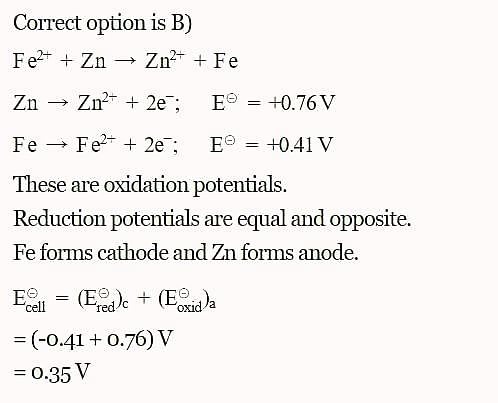
The standard reduction potentials E°, for the half reactions are as:
The emf for the cell reaction,
The correct order of equivalent conductance at infinite dilution of LiCl, NaCl and KCl is:
Saturated solution of KNO3 is used to make ‘salt-bridge’ because:
The specific conductances of four electrolytes in ohm−1cm−1 are given below. Which one offers higher resistance to passage of electric current?
The emf of the above cell is 0.2905 V. Equilibrium constant for the cell reaction is:
Given, standard electrode potentials, Fe2+ + 2e- →Fe, E° = -0.440V
Fe3+ +3e- → Fe, E° = -0.036V
The standard electrode potential (E°) for Fe3+ + e- → Fe2+ is:
Based on the data given blow strongest oxidizing agent will be:
The measured resistance of a conductance cell was 100 Ω. (MKCl = 74.5 gmol−1 and cell constant =1.25 cm−1). If the specific conductance in ohm−1 cm−1 is 125 × 10−x, then what is the value of x?
The solubility of a certain sparingly soluble substance MXn is nearly 1.4 × 10−4 M. If the solubility product is 1.1 × 10−11, what is the value of n?
The electrochemical cell shown below is a concentration cell.
M|M2+ (saturated solut ion of a sparingly so luble salt, MX2 || M2+ (0.001 mol dm–3)| M.
The emf of the cell depends on the difference in concentration of M2+ ions at the two electrodes. The emf of the cell at 298 is 0.059 V.
Q.
The value of ΔG (kJ mol-1) for the given cell is (take 1 F = 96500 C mol-1)
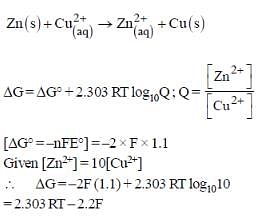
For the following cell,
Zn(s) | ZnSO4(aq) || CuSO4(aq) | Cu(s)
When the concentration of Zn2+ is 10 times the concentration of Cu2+, the expression for ΔG (in J mol-1) is [F is Faraday constant; R is gas constant; T is temperature; E° (cell) = 1.1 V]
An electrochemical cell consists of two half-cell reactions.
The mass of copper (in grams) dissolved on passing 0.5 A current for 1h is [Given, atomic mass of Cu is 63.6, F = 96500 C mol–1]
Using the following Latimer diagram for bromine, pH = 0;
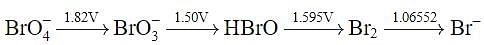
the species undergoing disproportionation is:
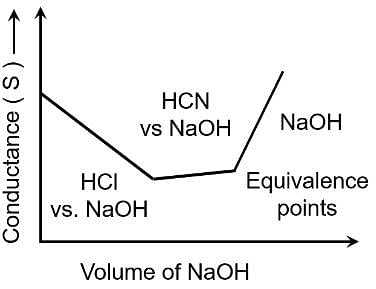
Conductometric titration curve of an equimolar mixture of HCl and HCN with NaOH (aq) can be given as
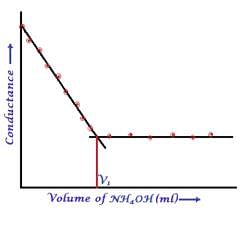
In a typical Conductometric titration of a strong acid with a weak base, the curve resembles:
|
108 videos|286 docs|123 tests
|