Test: Chemical Effects of Electric Current- 1 - Class 8 MCQ
20 Questions MCQ Test Science Class 8 - Test: Chemical Effects of Electric Current- 1
What type of water is a poor conductor of electricity?
What precaution should be taken when testing various liquids with the tester?
What is the process of depositing a layer of metal on another material using electricity called?
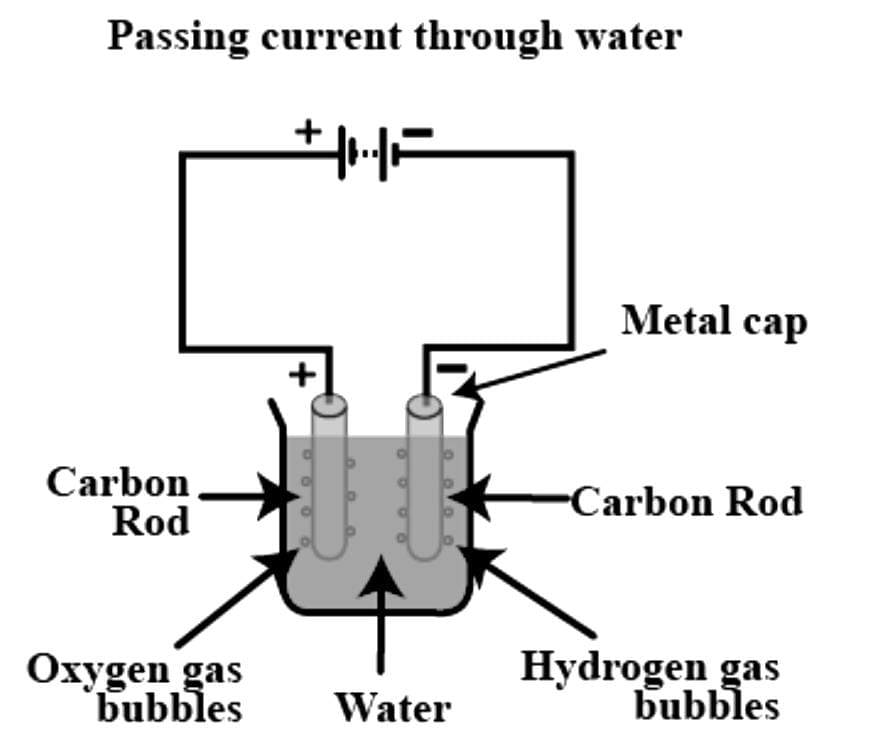
When electrodes are immersed in water and electricity is passed, the bubbles formed on the positive terminal is actually _______ gas.
Which of the following liquid is good conductor of electricity?
Which of the following gas is formed at the electrode connected to the negative terminal of the battery?
Which of the following is a chemical effect of electric current?
Which of the following is added to make the copper sulphate solution more conducting?
In LEDs, the longer lead (wire) is always connected to the _______ terminal
When electric current is passed through water, hydrogen is deposited over :
The substance through which current can flow easily are called
Assertion:Chromium plating is done on many objects like car parts, wheel rims and bath taps.
Reason:The chromed layer can be decorative, provide corrosion resistance, ease cleaning procedures, or increase surface hardness.
When electric current is passed through water, dissociation is indicated __________.
Chemical effect of electric current may cause :
A tester is used to check the conduction of electricity through two liquids, labeled A and B. It is found that the bulb of the tester glows brightly for liquid A while it glows very dimly for liquid B. You would conclude that:
What happens when an electric current passes through a conducting solution?
Tin cans are the tin electroplated on iron. These Tin cans are used to preserve food items. Why not iron cans used without Tin coating?
|
90 videos|278 docs|44 tests
|