Test: CSIR-NET Chemical Sciences Mock Test - 7 - UGC NET MCQ
30 Questions MCQ Test CSIR NET Exam Mock Test Series 2024 - Test: CSIR-NET Chemical Sciences Mock Test - 7
Pancreatic juice require which medium for their action?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
What is the insects which can transmit disease from one to another person are known as?
Directions: Answer the following questions by selecting the most appropriate option.
Cost price of 20 articles is equal to selling price of x articles. If the profit is 25%, then the value of x is
Select the correct mirror image of the given figure when the mirror is placed to the right of the figure.

Which number will replace the question mark (?) in the following series
6, 7, 11, 20,?
In the following question, some statements followed by some conclusions are given. Taking the given statements to be true even if they seem to be at variance from commonly known facts, read all the conclusions and then decide which of the given conclusions logically follows the given statements.
Statements:
Some clips are pins.
Some pins are pens.
Conclusions:
I. Some clips are pens.
II. No clips are pens.
III. Some pens are not pins.
Consider a dataset A with 55 distinct observations. A new dataset C is created by adding 2023 to all observations in dataset A. Which of the following is NOT true?
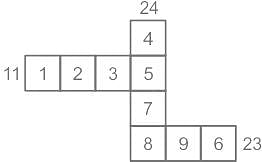
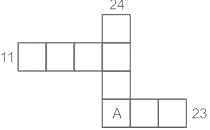
The squares in the following grid are filled with numbers 1 to 9 without any repetition, such that they add to 11 and 23 in the two horizontal rows and to 24 in the vertical column. What number appears in the square marked A?
Suppose a, b, c, d and e are five positive integers such that the values of a + b, b + c, c + d, d + e and e + 7 are same. Which of the following values is always same as a?
Two varieties of sugar A and B, costing Rs.40 per kg and Rs. 50 per kg, respectively were mixed before selling. The mixture containing 500 kg of A, was sold with a gain of 10% for Rs.88,000. How many kilograms of B did the sold mixture contain?
Dividing sixty by half and adding half of forty yields
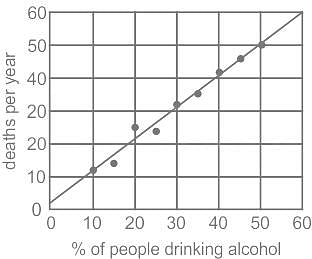
Given graph depicts the data of people drinking alcohol and deaths per year in nine villages. Which of the following can be definitely concluded from this graph?
Starting from the same point at the same time, A and B run on a 3600 m circular track with speeds of 4 m/s and 6 m/s, clockwise. After A completes the first round on the track, she reverses direction and runs anticlockwise. After how many seconds of starting the run would they cross for the first time?
Hypothetically, if the rotation rate of Earth about its axis is doubled, what would be the time difference (in standard seconds) between 30°E and 60°E longitudes in the equatorial region?
Which of the following is not done using column chromatography?
Antoine equation is a relation between
Which among the following is not a property of aromatic hydrocarbon:
Which of the following term describe saponification?
According to Newton’s law of viscosity, the maximum velocity of flow is at
The rate law for a reaction between the substances A and B is given by rate = K[A]n [B]m. On doubling the concentration of A and halving the concentration of B. What will be the ratio of the new rate of the earlier rate of the reaction?
Which type of chromatography is used for the structural analysis?
Melting is process which can be stated by the below statements except,
Which of the following is considered a useful alkali in saponification reactions?
The viscosity of a fluid is inversely proportional to the
For reaction system given below, volume is suddenly reduced to half of its value by increasing the pressure on it. If the reaction is of first order with respect to O2 and second order with respect to NO, what will be the change in the rate of reaction?
2NO(g) + O2(g) + 2NO2(g)
The energy required to rotate n-butane molecule about the carbon-carbon bond is called
Lye is used in soap-making. Lye is a concentrated solution of which ionic compound?
Which of the following is an example of bottom-up approach for the preparation of nanomaterials?