Test: Aldol Condensation - NEET MCQ
27 Questions MCQ Test Chemistry Class 12 - Test: Aldol Condensation
Only One Option Correct Type
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
Which is the major product in the following reaction?
Consider the following reaction,
Q.
Which of the labeiled C—C bond formation is not possible in the above reaction?

| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In a mixed aldoi condensation with ethanal as one aidehyde, which other from the following is expected to give maximum yield of cross condensation product?
Consider the following aldol condensation reaction,
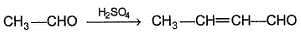
Q.
The nucleophile is
Identify the starting reagent needed to make the following compound by mixed aldol condensation.
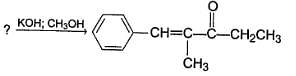
Which of the following could result as a product in the aldol condensation reaction?
What is the major final product of the following sequence of reaction?
One or More than One Options Correct Type
Direction (Q. Nos. 11-15) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q.
Successful mixed aldol condensation will be favoured
Which of the following is/are true regarding aldol condensation ?
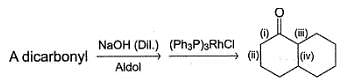
Which of the following indicated carbon-carbon bond can be formed via intramolecular aldoi condensation of a dicarbonyl compound?
Which compound (s) below can react via an intramolecular aldol condensation to give a six membered ring?
Statement Type
Direction (Q. Nos. 16-19) This section is based on Statement I and Statement II. Select the correct answer from the codes given below.
Q.
Statement I : In aldol condensation, enoiates are nucleophilic and reversibly attack the carbonyl carbon of aldehyde or ketone in the aldoi condensation.
Statement II : An aldoi on refluxing with dilute base gives back the carbonyl compound.
Statement I : When a mixture of ethanal and propanal is treated with aqueous Na2CO3, four aldol (excluding stereoisomers) are formed.
Statement II : In mixed aldol condensation, two self and two cross condensation products are always formed.
Statement I : When is treated with dilute base.
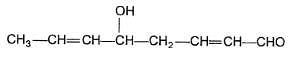 is formed.
is formed.
Statement II : In the given compound, γ-H is most acidic, forms the required enolate.
Statement I : In the reaction below,
A single aldol product is formed in 100% yield.
Statement II : Cross aldol product is formed as major product.
Comprehension Type
Direction (Q. Nos. 20-22) This section contains a paragraph, describing theory, experiments, data, etc.
Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
Aldol condensation is an important reaction in organic chemistry, particularly for the formation of carbon-carbon bonds. This reaction typically involves the enolate ion derived from an aldehyde or ketone reacting with another carbonyl compound to form a β-hydroxy aldehyde or β-hydroxy ketone. This product can undergo dehydration to form an α,β-unsaturated carbonyl compound. The reaction is usually catalyzed by a base, although acid-catalyzed versions are also known. The presence of at least one α-hydrogen in the reactant is crucial for the aldol condensation to occur. Acetaldehyde, for example, can undergo aldol condensation to yield 3-hydroxybutanal, which can then dehydrate to form crotonaldehyde.
Ques: In the aldol condensation reaction of acetaldehyde (CH₃CHO), the initial product formed before dehydration is:
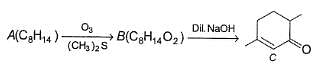
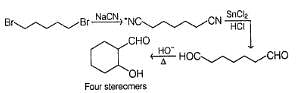
A is optically active and C is one of the several aldol possible in the above reaction.
Q.
Besides C, the other six membered cyclic aldol formed in the above reaction is

A is optically active and C is one of the several aldol possible in the above reaction.
Q.
The product B is stereomeric. If a mixture containing all stereoisomers of B is treated with excess of LiAIH4 followed by the acidification will give how many different isomeric diols ?
One Integer Value Correct Type
Direction (Q. Nos. 23-27) This section contains 5 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
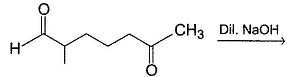
Consider the following aldol condensation reaction,
Butanone + NaOH (Dil.) → Aldols
Q.
How many different isomers (including stereoisomers) are formed above?
Consider the following cross-aldol condensation reaction,
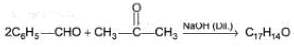

Q.
How many different isomeric X are formed?
Consider the following modified aldol condensation,
Q.
How many ethanal, at the most, will react with one molecule of nitromethane?
Consider the following sequence of reaction,
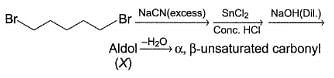
Q.
How many different aldol isomers of X are formed?
Consider the following sequence of reaction,
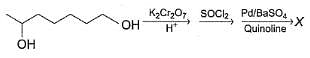
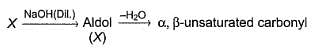
Q.
If all undehydrated aldols (X) formed above by intramolecular aldol condensation are considered, how many pairs of enantiomers are formed?
|
100 videos|282 docs|123 tests
|