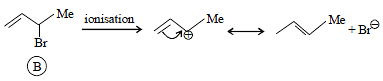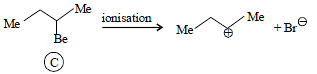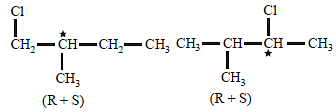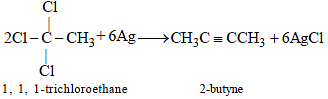Test: JEE Main 35 Year PYQs- Haloalkanes & Haloarenes- 1 - JEE MCQ
18 Questions MCQ Test Chapter-wise Tests for JEE Main & Advanced - Test: JEE Main 35 Year PYQs- Haloalkanes & Haloarenes- 1
Bottles containing C6H5I and C6H5CH2I lost their originallabels. They were labelled A and B for testing. A and B wereseparately taken in test tubes and boiled with NaOH solution.The end solution in each tube was made acidic with dilute HNO3 and then some AgNO3 solution was added. Substance B gave a yellow precipitate. Which one of the followingstatements is true for this experiment ? [2003]
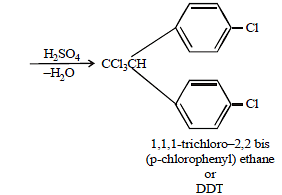
The compound formed on heating chlorobenzene withchloral in the presence of concentrated sulphuric acid, is [2004]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Tertiary alkyl halides are practically inert to substitution bySN2 mechanism because of [2005]
Alkyl halides react with dialkyl copper reagents to give [2005]
Elimination of bromine from 2-bromobutane results in theformation of – [2005]
Phenyl magnesium bromide reacts with methanol to give [2006]
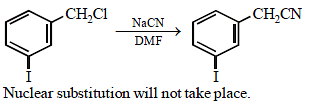
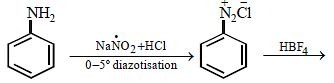
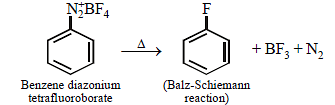
Fluorobenzene (C6H5F) can be synthesized in the laboratory [2006]
Reaction of trans 2-phenyl-1-bromocyclopentane onreaction with alcoholic KOH produces [2006]
The structure of the major product formed in the following reaction
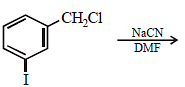 is
is
Which of the following is the correct order of decreasingSN2 reactivity? [2007]
(X is a halogen)
The organic chloro compound, which shows completesterochemical inversion during a SN2 reaction, is [2008]
Consider the following bromides :
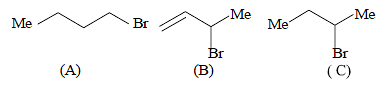
The correct order of SN1 reactivity is [2010]
How many chiral compounds are possible onmonochlorination of 2- methyl butane ? [2012]
What is DDT among the following ? [2012]
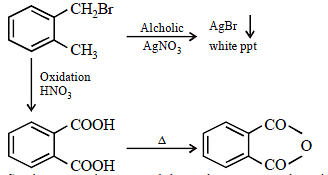
CompoundCompound (A), C8H9Br, gives a white precipitate when warmed with alcoholic AgNO3. Oxidation of (A) gives an
acid (B), C8H6O4. (B) easily forms anhydride on heating. Identify the compound (A). [JEE M 2013]
In SN2 reactions, the correct order of reactivity for thefollowing compounds: [JEE M 2014]
CH3Cl,CH3CH2Cl, (CH3)2CHCl and ( CH3)3 CCl is:
The major organic compound formed by the reaction of 1, 1, 1-trichloroethane with silver powder is: [JEE M 2014]
The synthesis of alkyl fluorides is best accomplished by : [JEE M 2015]
|
446 docs|930 tests
|
|
446 docs|930 tests
|


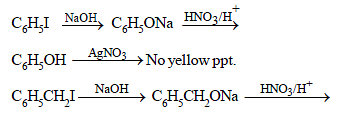
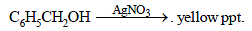
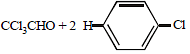
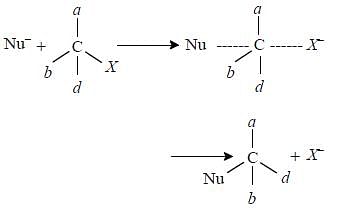
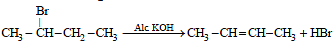
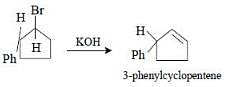 It follows E2 mechanism. Hughes and Ingold proposed that bimolecular elimination reactions take place when the two groups to be eliminated are trans and lie in one plane with the two carbon atoms to which they are attached i.e. E2 reactions are stereoselectively trans.
It follows E2 mechanism. Hughes and Ingold proposed that bimolecular elimination reactions take place when the two groups to be eliminated are trans and lie in one plane with the two carbon atoms to which they are attached i.e. E2 reactions are stereoselectively trans.