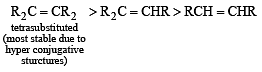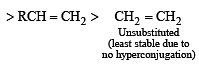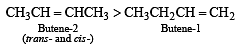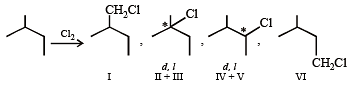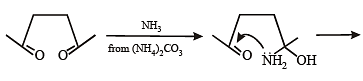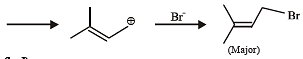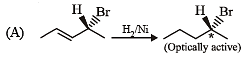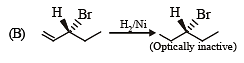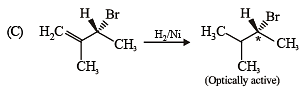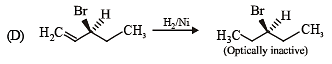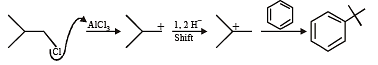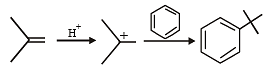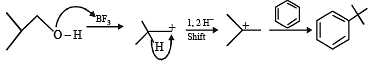JEE Exam > JEE Tests > 35 Years Chapter wise Previous Year Solved Papers for JEE > Test: MCQs (One or More Correct Option): Hydrocarbons | JEE Advanced - JEE MCQ
Test: MCQs (One or More Correct Option): Hydrocarbons | JEE Advanced - JEE MCQ
Test Description
7 Questions MCQ Test 35 Years Chapter wise Previous Year Solved Papers for JEE - Test: MCQs (One or More Correct Option): Hydrocarbons | JEE Advanced
Test: MCQs (One or More Correct Option): Hydrocarbons | JEE Advanced for JEE 2024 is part of 35 Years Chapter wise Previous Year Solved Papers for JEE preparation. The Test: MCQs (One or More Correct Option): Hydrocarbons | JEE Advanced questions and answers have been
prepared according to the JEE exam syllabus.The Test: MCQs (One or More Correct Option): Hydrocarbons | JEE Advanced MCQs are made for JEE 2024 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: MCQs (One or More Correct Option): Hydrocarbons | JEE Advanced below.
Solutions of Test: MCQs (One or More Correct Option): Hydrocarbons | JEE Advanced questions in English are available as part of our 35 Years Chapter wise Previous Year Solved Papers for JEE for JEE & Test: MCQs (One or More Correct Option): Hydrocarbons | JEE Advanced solutions in
Hindi for 35 Years Chapter wise Previous Year Solved Papers for JEE course. Download more important topics, notes, lectures and mock
test series for JEE Exam by signing up for free. Attempt Test: MCQs (One or More Correct Option): Hydrocarbons | JEE Advanced | 7 questions in 10 minutes | Mock test for JEE preparation | Free important questions MCQ to study 35 Years Chapter wise Previous Year Solved Papers for JEE for JEE Exam | Download free PDF with solutions
*Multiple options can be correct
Test: MCQs (One or More Correct Option): Hydrocarbons | JEE Advanced - Question 1
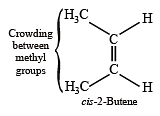
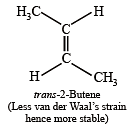
Which one of the following has the smallest heat of hydrogenation per mole? (1993)
Detailed Solution for Test: MCQs (One or More Correct Option): Hydrocarbons | JEE Advanced - Question 1
*Multiple options can be correct
Test: MCQs (One or More Correct Option): Hydrocarbons | JEE Advanced - Question 2
Toluene, when treated with Br2/Fe, gives p-bromotoluene as the major product because CH3 group (1999 - 3 Marks)
Detailed Solution for Test: MCQs (One or More Correct Option): Hydrocarbons | JEE Advanced - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
*Multiple options can be correct
Test: MCQs (One or More Correct Option): Hydrocarbons | JEE Advanced - Question 3
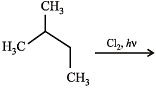 N(isomeric products) ; C5H11Cl
N(isomeric products) ; C5H11Cl
 (isomeric products) Identify N and M (2006 - 5M,)
(isomeric products) Identify N and M (2006 - 5M,)
Identify N and M (2006 - 5M, –1)
 N(isomeric products) ; C5H11Cl
N(isomeric products) ; C5H11Cl (isomeric products) Identify N and M (2006 - 5M,)
(isomeric products) Identify N and M (2006 - 5M,)
Detailed Solution for Test: MCQs (One or More Correct Option): Hydrocarbons | JEE Advanced - Question 3
*Multiple options can be correct
Test: MCQs (One or More Correct Option): Hydrocarbons | JEE Advanced - Question 4
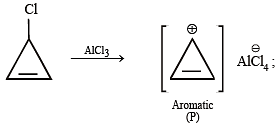
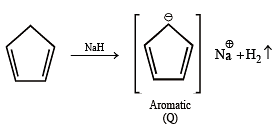
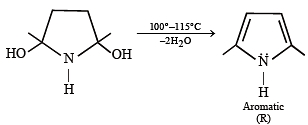
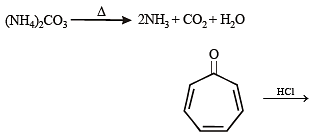
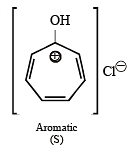
Among P, Q, R and S, the aromatic compound(s) is/are (JEE Advanced 2013-I)
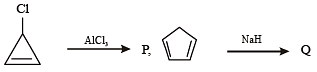

Detailed Solution for Test: MCQs (One or More Correct Option): Hydrocarbons | JEE Advanced - Question 4
*Multiple options can be correct
Test: MCQs (One or More Correct Option): Hydrocarbons | JEE Advanced - Question 5
In the following reaction, the major product is (JEE Adv. 2015)
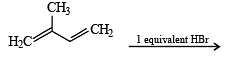
Detailed Solution for Test: MCQs (One or More Correct Option): Hydrocarbons | JEE Advanced - Question 5
*Multiple options can be correct
Test: MCQs (One or More Correct Option): Hydrocarbons | JEE Advanced - Question 6
Compound(s) that on hydrogenation produce(s) optically inactive compound(s) is (are) (JEE Adv. 2015)
Detailed Solution for Test: MCQs (One or More Correct Option): Hydrocarbons | JEE Advanced - Question 6
*Multiple options can be correct
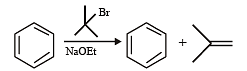
Test: MCQs (One or More Correct Option): Hydrocarbons | JEE Advanced - Question 7
Among th e followin g, r eaction(s) wh ich gives(give) tert-butyl benzene as the major product is(are) (JEE Adv. 2016)
Detailed Solution for Test: MCQs (One or More Correct Option): Hydrocarbons | JEE Advanced - Question 7
|
347 docs|185 tests
|
Information about Test: MCQs (One or More Correct Option): Hydrocarbons | JEE Advanced Page
In this test you can find the Exam questions for Test: MCQs (One or More Correct Option): Hydrocarbons | JEE Advanced solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: MCQs (One or More Correct Option): Hydrocarbons | JEE Advanced, EduRev gives you an ample number of Online tests for practice
|
347 docs|185 tests
|
Download as PDF