Test: MCQs (One or More Correct Option): Thermodynamics | JEE Advanced - JEE MCQ
7 Questions MCQ Test 35 Years Chapter wise Previous Year Solved Papers for JEE - Test: MCQs (One or More Correct Option): Thermodynamics | JEE Advanced
Identify the intensive quantities from the following: (1993 - 1 Mark)
The following is (are) endothermic reaction(s): (1999 - 3 Marks)
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Among the following the state function(s) is (are) (2009)
Among the following, the intensive property is (properties are) (2010)
For an ideal gas, consider only P–V work in going from an initial state X to the final state Z. The final state Z can be reached by either of the two paths shown in the figure.
Which of the following choice(s) is (are) correct? [Take ΔS as change in entropy and w as work done]. (2012)
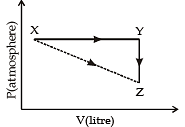
The reversible expansion of an ideal gas under adiabatic and isothermal conditions is shown in the figure. Which of the following statement(s) is (are) correct ?

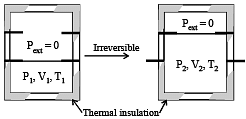
An ideal gas in a thermally insulated vessel at internal pressure = P1, volume = V1 and absolute temperature = T1 expands irreversibly against zero external pressure, as shown in the diagram. The final internal pressure, volume and absolute temperature of the gas are P2, V2 and T2, respectively. For this expansion, (JEE Adv. 2014)
|
347 docs|185 tests
|
|
347 docs|185 tests
|












