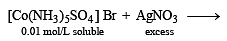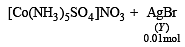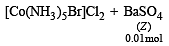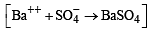JEE Advanced (Single Correct MCQs): Some Basic Concepts of Chemistry - JEE MCQ
28 Questions MCQ Test Chapter-wise Tests for JEE Main & Advanced - JEE Advanced (Single Correct MCQs): Some Basic Concepts of Chemistry
27 g of Al will react completely with how many grams of oxygen?(1978)
A compound was found to contain nitrogen and oxygen in the ratio 28 gm and 80 gm respectively. The formula of compound is (1978)
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The largest number of molecules is in (1979)
The total number of electrons in one molecule of carbon dioxide is
A gaseous mixture contains oxygen and nitrogen in the ratio of 1 : 4 by weight. Therefore the ratio of their number of molecules is (1979)
2.76 g of silver carbonate on being strongly heated yields a residue weighing (1979)
M is molecular weight of KMnO4. The equivalent weight of KMnO4 when it is converted into K2MnO4 is (1980)
If 0.50 mole of BaCl2 is mixed with 0.20 mol of Na3PO4, the maximum number of moles of Ba3(PO4)2 that can be formed is (1981 - 1 Mark)
One mole of N2H4 loses ten moles of electrons to form a new compound Y. Assuming that all the nitrogen appears in the new compound, what is the oxidation state of nitrogen in Y? (There is no change in the oxidation state of hydrogen). (1981 - 1 Mark)
The oxidation number of carbon in CH2O is (1982 - 1 Mark)
A molal solution is one that contains one mole of a solute in: (1986 - 1 Mark)
The brown ring complex compound is for mulated as [Fe(H2O)5(NO)]SO4. The oxidation state of iron is :
The equivalent weight of MnSO4 is half of its molecular weight when it is converted to : (1988 - 1 Mark)
In which mode of expression, the concentration of a solution remains independent of temperature? (1988 - 1 Mark)
The oxidation number of phosphorus in Ba(H2PO2)2 is : (1990 - 1 Mark)
The oxidation states of the most electronegative element in the products of the reaction, BaO2 with dil. H2SO4 is (1991 - 1 Mark)
For the redox reaction :
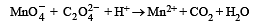
the correct coefficients of the reactants for the balanced reaction are (1992 - 1 Mark)
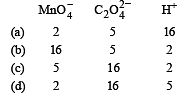
The normality of 0.3 M phosphorous acid (H3PO3) is, (1999 - 2 Marks)
The oxidation number of sulph ur in S8 , S2F2, H2 S respectively, are (1999 - 2 Marks)
Amongst the following identify the species with an atom in +6 oxidation state (2000S)
The reaction, 3ClO-(aq) → ClO 3- (aq) + 2Cl-(aq), is an example of (2001S)
An aqueous solution of 6.3 g oxalic acid dihydrate is made up to 250 ml. The volume of 0.1 N NaOH required to completely neutralize 10 ml of this solution is (2001S)
In the standardization of Na2S2O3 using K2Cr 2O7 by iodometry, the equivalent weight of K2Cr2O7 is (2001S)
How many moles of electron weigh one kilogram? (2002S)
Which has maximum number of atoms? (2003S)
Mixture X = 0.02 mol of [Co(NH3)5SO4]Br and 0.02 mol of [Co(NH3)5Br]SO4 was prepared in 2 litre of solution. (2003S)
1 litre of mixture X + excess AgNO3 —→ Y.
1 litre of mixture X + excess BaCl2 —→ Z
No. of moles of Y and Z are
The pair of the compounds in which both the metals are in the highest possible oxidation state is (2004S)
Consider a titration of potassium dichromate solution with acidified Mohr's salt solution using diphenylamine as indicator. The number of moles of Mohr's salt required per mole of dichromate is (2007)
|
447 docs|930 tests
|
|
447 docs|930 tests
|



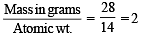
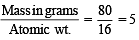
 × 6.02 × 1023 molecules of N2O5
× 6.02 × 1023 molecules of N2O5  molecules of nitrogen
molecules of nitrogen molecules of nitrogen
molecules of nitrogen
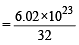 moleculesof oxygen
moleculesof oxygen = 7 : 32
= 7 : 32
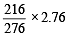 = 2.16g of Ag
= 2.16g of Ag
 = M [∵ Mol. wt. = M]
= M [∵ Mol. wt. = M]
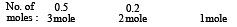
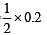 = 0.1 mol.
= 0.1 mol.
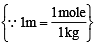

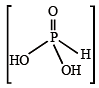

 = 25.2 g/L
= 25.2 g/L


 = 40 ml.
= 40 ml.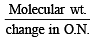

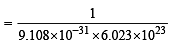

 × 6.023 × 1023 = 2 × 6.023 × 1023 atom
× 6.023 × 1023 = 2 × 6.023 × 1023 atom × 6.023 × 1023 = 6.023 × 1023 atom = 1 mole atoms
× 6.023 × 1023 = 6.023 × 1023 atom = 1 mole atoms × 6.023 × 1023 = 6.023 × 1023 atom = 1 mole atoms
× 6.023 × 1023 = 6.023 × 1023 atom = 1 mole atoms × 6.023 × 1023 = 6.023 × 1023 atom = 1 mole atoms
× 6.023 × 1023 = 6.023 × 1023 atom = 1 mole atoms