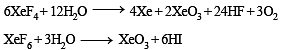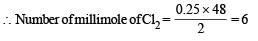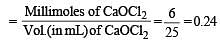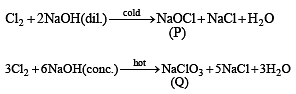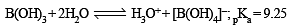Test: Comprehension Based Questions: The p-Block Elements - JEE MCQ
17 Questions MCQ Test 35 Years Chapter wise Previous Year Solved Papers for JEE - Test: Comprehension Based Questions: The p-Block Elements
PASSAGE - 1
The noble gases have closed-shell electronic configuration and are monoatomic gases under normal conditions. The low boiling points of the lighter noble gases are due to weak dispersion forces between the atoms and the absence of other interatomic interactions.
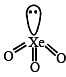
The direct reaction of xenon with fluorine leads to a series of compounds with oxidation numbers +2, +4 and +6. XeF4 reacts violently with water to given XeO3. The compounds of xenon exhibit rich stereochemistry and their geometries can be deduced considering the total number of electron pairs in the valence shell.
Q. Argon is used in arc welding because of its
PASSAGE - 1
The noble gases have closed-shell electronic configuration and are monoatomic gases under normal conditions. The low boiling points of the lighter noble gases are due to weak dispersion forces between the atoms and the absence of other interatomic interactions.
The direct reaction of xenon with fluorine leads to a series of compounds with oxidation numbers +2, +4 and +6. XeF4 reacts violently with water to given XeO3. The compounds of xenon exhibit rich stereochemistry and their geometries can be deduced considering the total number of electron pairs in the valence shell.
Q. The structure of XeO3 is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
PASSAGE - 1
The noble gases have closed-shell electronic configuration and are monoatomic gases under normal conditions. The low boiling points of the lighter noble gases are due to weak dispersion forces between the atoms and the absence of other interatomic interactions.
The direct reaction of xenon with fluorine leads to a series of compounds with oxidation numbers +2, +4 and +6. XeF4 reacts violently with water to given XeO3. The compounds of xenon exhibit rich stereochemistry and their geometries can be deduced considering the total number of electron pairs in the valence shell.
Q. XeF4 and XeF6 are expected to be
PASSAGE - 2
There are some deposits of nitrates and phosphates in earth’s crust. Nitrates are more soluble in water. Nitrates are difficult to reduce under the laboratory conditions but microbes do it easily.
Ammonia forms large number of complexes with transition metal ions. Hybridization easily explains the ease of sigma donation capability of NH3 and PH3. Phosphine is a flammable gas and is prepared from white phosphorous.
Q. Among the following, the correct statement is
PASSAGE - 2
There are some deposits of nitrates and phosphates in earth’s crust. Nitrates are more soluble in water. Nitrates are difficult to reduce under the laboratory conditions but microbes do it easily.
Ammonia forms large number of complexes with transition metal ions. Hybridization easily explains the ease of sigma donation capability of NH3 and PH3. Phosphine is a flammable gas and is prepared from white phosphorous.
Q. Among the following, the correct statement is
PASSAGE - 2
There are some deposits of nitrates and phosphates in earth’s crust. Nitrates are more soluble in water. Nitrates are difficult to reduce under the laboratory conditions but microbes do it easily.
Ammonia forms large number of complexes with transition metal ions. Hybridization easily explains the ease of sigma donation capability of NH3 and PH3. Phosphine is a flammable gas and is prepared from white phosphorous.
Q. White phosphorus on reaction with NaOH gives PH3 as one of the products. This is a
PASSAGE - 3
Bleaching powder and bleach solution are produced on a large scale and used in several household products. The effectiveness of bleach solution is often measured by iodometry.
Q. Bleaching powder contains a salt of an oxoacid as one of its components. The anhydride of that oxoacid is
PASSAGE - 3
Bleaching powder and bleach solution are produced on a large scale and used in several household products. The effectiveness of bleach solution is often measured by iodometry.
Q. 25 mL of household solution was mixed with 30 mL of 0.50 M KI and 10 mL of 4N acetic acid. In the titration of the liberated iodine, 48 mL of 0.25 N Na2S2O3 was used to reach the end point. The molarity of the household bleach solution is
PASSAGE - 4
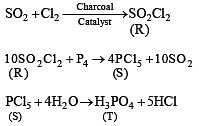
The reactions of Cl2 gas with cold-dilute and hot-concentrated NaOH in water give sodium salts of two (different) oxoacids of chlorine, P and Q, respectively. The Cl2 gas reacts with SO2 gas, in presence of charcoal, to give a product R. R reacts with white phosphorus to give a compound S. On hydrolysis, S gives an oxoacid of phosphorus, T.
Q. P and Q, respectively, are the sodium salts of
PASSAGE - 4
The reactions of Cl2 gas with cold-dilute and hot-concentrated NaOH in water give sodium salts of two (different) oxoacids of chlorine, P and Q, respectively. The Cl2 gas reacts with SO2 gas, in presence of charcoal, to give a product R. R reacts with white phosphorus to give a compound S. On hydrolysis, S gives an oxoacid of phosphorus, T.
Q. R, S and T respectively, are
This question contains STATEMENT-1 (Assertion/ Statement ) and STATEMENT-2 (Reason/Explanation) and has 4 choices (a), (b), (c) and (d) out of which ONLY ONE is correct.
Q.
Statement-1 : Although PF5 , PCl5 and PBr5 are known,the pentahalides of nitrogen have not been observed Statement-2 : Phosphorus has lower electronegativity than nitrogen.
Statement-1 : F atom has less electron affinity than Cl atom.
Statement-2 : Additional electrons are repelled more effectively by 3p electrons in Cl atom than by 2p electrons in F atom.
Statement-1 : Al(OH)3 is amphoteric in nature
Statement-2 : Al–O and O–H bonds can be broken with equal ease in Al(OH)3.
Statement-1 : Between SiCl4 and CCl4, only SiCl4 reacts with water.
Statement-2 : SiCl4 is ionic and CCl4 is covalent.
Statement-1 : In water, orthoboric acid behaves as a weak monobasic acid. because
Statement-2 : In water, orthoboric acid acts as a proton donor.
Statement-1 : Boron always forms covalent bond. because
Statement-2 : The small size of B3+ favours formation of covalent bond.
Statement-1 : Pb+4 compounds are stronger oxidising agents than Sn4+ compounds
Statement-2 : The higher oxidation states for the group 14 elements are more stable for the heavier members of the group due to 'inert pair effect'.
|
327 docs|185 tests
|
|
327 docs|185 tests
|