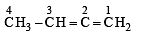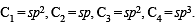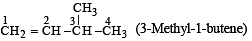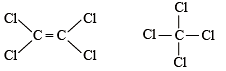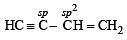JEE Advanced (Single Correct MCQs): Organic Chemistry - Some Basic Principles & Technique - JEE MCQ
30 Questions MCQ Test Chapter-wise Tests for JEE Main & Advanced - JEE Advanced (Single Correct MCQs): Organic Chemistry - Some Basic Principles & Technique
The bond order of in dividual carbon -carbon bonds in benzene is
Molecule in which the distance between the two adjacent carbon atoms is largest is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The compound which is not isomeric with diethyl ether is
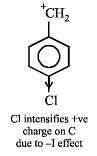
Among the following, the compound that can be most readily sulphonated is
The compound 1, 2-butadiene has
Which of the following compounds will exhibit cis-trans (geometrical) isomerism?
The IUPAC name of the compound having the formula is:
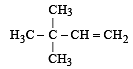
An isomer of ethanol is :
Out of the following compounds, which will have a zero dipole moment?
The bond between carbon atom (1) and carbon atom (2) in compound 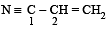 involves the hybrids as
involves the hybrids as
The IUPAC name of the compound
CH2 = CH – CH (CH3)2 is
The number of isomers of C6H14 is
The Cl—C—Cl angle in 1,1,2,2-tetrachloroethene and tetrachloromethane respectively will be about
The compound which has one isopropyl group is :
The C–H bond distance is the longest in :
The number of sigma and pi-bonds in 1-butene-3-yne are :
In CH3CH2OH, the bond that undergoes heterolytic cleavage most readily is
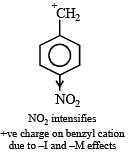
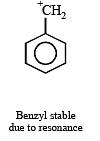
The compound which gives the most stable carbonium ion on dehydration is :
The hybridization of carbon atoms in C–C single bond of HC ≡ C – CH = CH2 is
The products of combustion of an aliphatic thiol (RSH) at 298 K are
Isomers which can be inter converted th rough rotation around a single bond are
The structure 
Allyl isocyanide has :
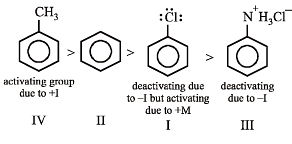
Arrange in order of decreasing trend towards SE reactions :


Most stable carbonium ion is :
In the following compounds,
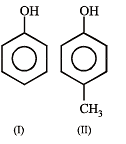

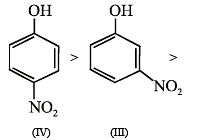
The order of acidity is :
Arrange the following compounds in order of increasing dipole moment.
Toluene (I)
m-dichlorobenzene (II)
o-dichlorobenzene (III)
p-dichlorobenzene (IV)
How many optically active stereoisomers are possible for butane-2, 3-diol?
In the compound CH2 = CH–CH2–CH2–C ≡ CH, the C2–C3 bond is of the type,
The optically active tartaric acid is named as D – (+) – tartaric acid because it has a positive
|
446 docs|930 tests
|
|
446 docs|930 tests
|