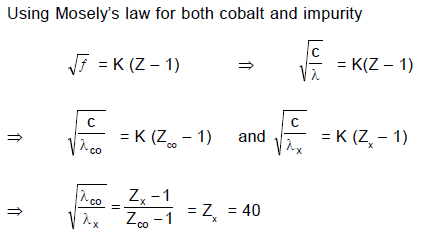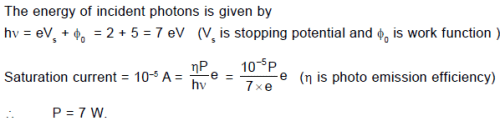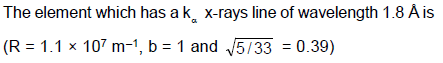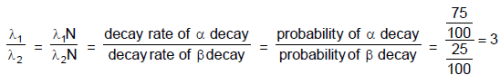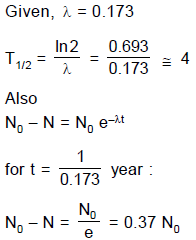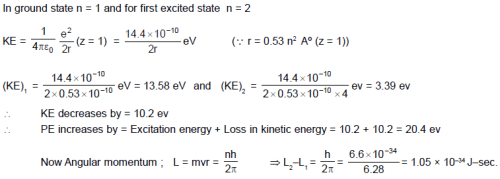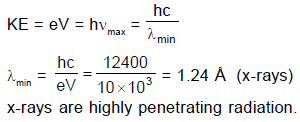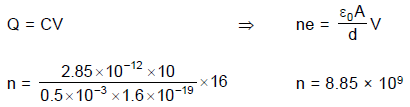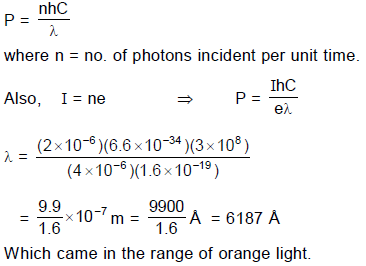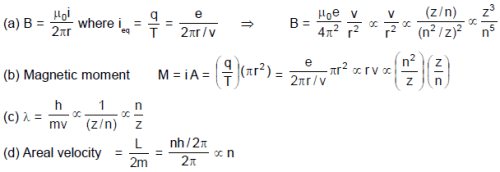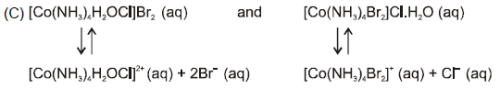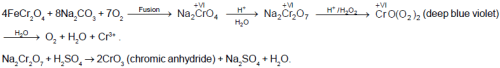JEE Advanced Test- 12 - JEE MCQ
30 Questions MCQ Test Mock Tests for JEE Main and Advanced 2025 - JEE Advanced Test- 12
A cobalt (atomic no. = 27) target is bombarded with electrons, and the wavelengths of its characteristic x-ray spectrum are measured. A second weak characteristic spectrum is also found, due to an impurity in the target. The wavelengths of the Kα lines are 225.0 pm (cobalt) and 100.0 pm (impurity). Atomic number of the impurity is (take b = 1)
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
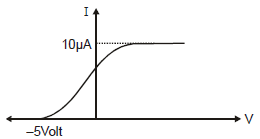
In the photoelectric experiment, if we use a monochromatic light, the I - V curve is as shown. If work function of the metal is 2eV, estimate the power of light used. (Assume efficiency of photo emission = 10–3%, i.e. number of photoelectrons emitted are 10–3% of number of photons incident on metal.)

In the following question, a Statement of Assertion (A) is given followed by a corresponding Reason(R) just below it.Read the Statement carefully and mark the correct answer-
Assertion(A): Protostele is the simplest type of stele.
Reason (R): Siphonostele has pith in the centre.
A 10 μ F capacitor and a 20 μ F capacitor are connected in series across 200 V supply line. The charged capacitors are then disconnected from the line and reconnected with their positive plates together and negative plates together and no external voltage is applied. What is the potential difference across each capacitor?
A metallic charged ring is placed in a uniform magnetic field with its plane perpendicular to the field. If the magnitude of field starts increasing with time, then :
An electron in hydrogen atom makes a transition n1 → n2 where n1 and n2 are principal quantum numbers of the two states. Assuming Bohr's model to be valid the time period of the electron in the initial state is eight times that in the final state. The possible values of n1 and n2 are
The decay constant of a radio active substance is 0.173 (years)-1. Therefore :
In an x-ray tube the voltage applied is 20 kV. The energy required to remove an electron from L shell is 19.9 keV. In the x-rays emitted by the tube (Use hc = 12420 eVÅ)
When a hydrogen atom is excited from ground state to first excited state then
Consider the following four electrodes,
P = Cu2+ (0.0001 M) | Cu(s)
Q = Cu2+ (0.1 M) | Cu(s)
R = Cu2+ (0.01 M) | Cu(s)
S = Cu2+ (0.001 M) | Cu(s)
If the standard reduction potential of Cu2+/Cu is +0.34 V, the reduction potentials in volts of the above electrodes follow the order
Statement-1 : In process of photoelectric emission, all emitted electrons donot have same kinetic energy.
Statement-2 : If radiation falling on photosensitive surface of a metal consists of different wavelengths, then energy acquired by electrons absorbing photons of different wavelengths shall be different.
Statement-1 : When a beam of highly energetic neutrons is incident on a tungsten target, no X-rays will be produced.
Statement-2 : Neutrons do not exert any electrostatic force on electrons or nucleus of an atom.
Statement-1 : When cathode rays (accelerated with high voltage 10 kVolts) strikes a hard metallic surface, highly penetrating radiation is obtained.
Statement-2 : Due to conversion of electron's kinetic energy into photon's energy, the shortest wavelength limit of X-rays produced is inversely proportional to the accelerating voltage.
Statement-1 : Heavy nuclides tend to have more number of neutrons than protons.
Statement-2 : In heavy nuclei, as there is coloumbic repulsion between protons, so excess of neutrons are preferable.
An experimental setup of verification of photoelectric effect is shown in the diagram. The voltage across the electrodes is measured with the help of an ideal voltmeter and which can be varied by moving jockey ‘J’ on the potentiometer wire. The battery used in potentiometer circuit is of 20 V and its internal resistance is 2?. The resistance of 100 cm long potentiometer wire is 8?.
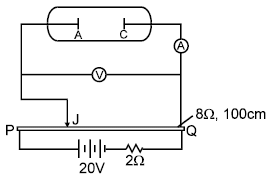
The photocurrent is measured with the help of an ideal ammeter. Two plates of potassium oxide of area 50 cm2 at separation 0.5 mm are used in the vacuum tube. Photo current in the circuit is very small so we can treat potentiometer circuit an independent circuit.
The wavelengths of various colours is as follows :

Q. The number of electrons appeared on the surface of the cathode plate, when the jockey is connected at the end ‘P’ of the potentiometer wire. Assume that no radiation is falling on the plates.
An experimental setup of verification of photoelectric effect is shown in the diagram. The voltage across the electrodes is measured with the help of an ideal voltmeter and which can be varied by moving jockey ‘J’ on the potentiometer wire. The battery used in potentiometer circuit is of 20 V and its internal resistance is 2?. The resistance of 100 cm long potentiometer wire is 8?.

The photocurrent is measured with the help of an ideal ammeter. Two plates of potassium oxide of area 50 cm2 at separation 0.5 mm are used in the vacuum tube. Photo current in the circuit is very small so we can treat potentiometer circuit an independent circuit.
The wavelengths of various colours is as follows :

Q. It is found that ammeter current remains unchanged (2μA) even when the jockey is moved from the end ‘P’ to the middle point of the potentiometer wire. Assuming all the incident photons eject electron and the power of the light incident is 4 × 10–6 W. Then the colour of the incident light is
An experimental setup of verification of photoelectric effect is shown in the diagram. The voltage across the electrodes is measured with the help of an ideal voltmeter and which can be varied by moving jockey ‘J’ on the potentiometer wire. The battery used in potentiometer circuit is of 20 V and its internal resistance is 2?. The resistance of 100 cm long potentiometer wire is 8?.

The photocurrent is measured with the help of an ideal ammeter. Two plates of potassium oxide of area 50 cm2 at separation 0.5 mm are used in the vacuum tube. Photo current in the circuit is very small so we can treat potentiometer circuit an independent circuit.
The wavelengths of various colours is as follows :

Q. When other light falls on the anode plate the ammeter reading remains zero till, jockey is moved from the end P to the middle point of the wire PQ. Thereafter the deflection is recorded in the ammeter. The maximum kinetic energy of the emitted electron is
Consider the reaction,
Cl2 (aq) + H2S (aq) → S(s) + 2H+(aq) + 2Cl–(aq)
The rate equation for this reaction is, rate = k [Cl2] [H2S]
Which of these mechanisms is/are consistent with this rate equation?
(a). Cl2 + H2S → H+ + Cl– + Cl+ + HS– (slow)
Cl+ + HS– → H+ + Cl– + S (fast)
(b). H2S  H+ + HS– (fast equilibrium)
H+ + HS– (fast equilibrium)
Cl2 + HS– → 2Cl– + H+ + S (slow)
The half life period of a first order chemical reaction is 6.93 minutes. The time required for the completion of 99% of the chemical reaction will be (log 2 = 0.301)
Consider a reaction, aG + bH → Products.
When concentration of both the reactants G and H is doubled, the rate increases by eight times. However, when the concentration of G is doubled keeping the concentration of H fixed, the rate is doubled. The overall order of the reaction is:
Using Bohr’s model for H–like atom, match the following. Here n = orbit number, Z = nuclear charge, m = mass of electron
Column-I Column-II
(A) Due to revolving electron, the magnetic field produced (p) n–5
at its centre is proportional to
(B) Magnetic moment of revolving electron is proportional to (q) n
(C) De-Broglie wave length of revolving electron is proportional to (r) Z3
(D) Areal velocity of revolving electron about nucleus is (s) independent of Z
proportional to
(t) m–1
For a reaction A + B → C + D if the concentration of A is doubled without altering the concentration of B, the rate gets doubled. If the concentration of B is increased by nine times without altering the concentration of A, the rate gets tripled. The order of the reaction is
When a bio chemical reaction is carried out in laboratory out side the human body in the absence of enzyme, then the rate of reaction obtained is 10–6 times, than activation energy of
reaction in the presence of enzyme is:
'Spin only' magnetic moment of Ni in [Ni(dmg)2] is same as that found in :
Which of the following complexes shows ionisation as well as hydrate isomerism ?

Which of the following statements is true for the compounds [X], [Y] and [Z] ?
Maximum number of electrons in a subshell with l = 3 and n = 4 is
|
357 docs|148 tests
|
|
357 docs|148 tests
|