Test: Periodic Trends in Properties of Elements (May 11) - JEE MCQ
10 Questions MCQ Test Daily Test for JEE Preparation - Test: Periodic Trends in Properties of Elements (May 11)
What are the two radii shown as 'a' and 'b' in the figure known as?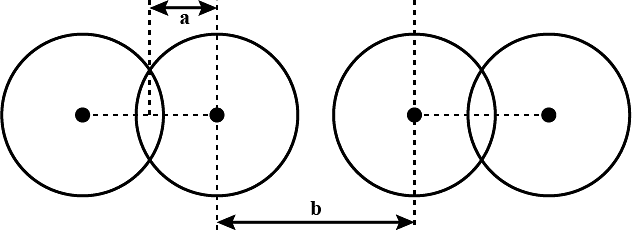

Which of the following statements regarding the variation of atomic radii in the periodic table is not true?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
K+ and Cl- ions are isoelectronic. Which of the statements is not correct?
What is the order of successive ionisation enthalpies?
Which of the following elements will have highest second ionisation enthalpy?
Of the metals Be, Mg., Ca and Sr of group 2 in the periodic table, the least ionic chloride will be formed by
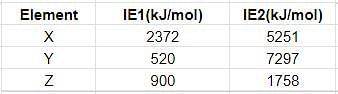
Few elements are matched with their successive ionisation energies. Identify the elements.
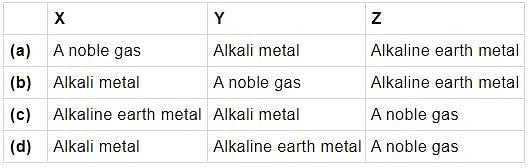
The electronic states X and Y of an atom are depicted below:
X : 1s2 2s2 2p6 3s1
Y : 1s2 2s2 2p6 3s2 3p6 4s1
Which of the following statements is not correct?
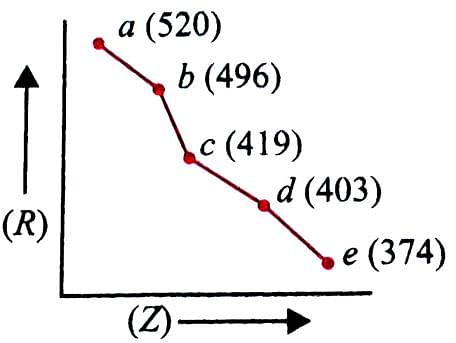
In the given graph, a periodic property (R) is plotted against atomic numbers (Z) of the elements. Which property is shown in the graph and how is it correlated with reactivity of the elements?
Which of the following will have lowest electron affinity?
|
360 tests
|


















