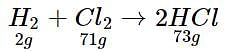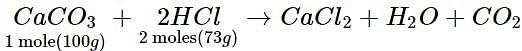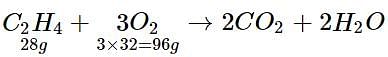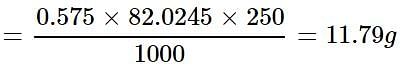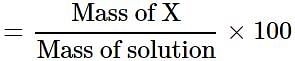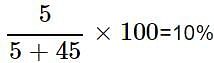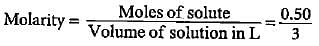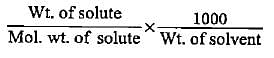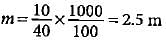Test: Stoichiometry and Stoichiometric Calculations (April 8) - JEE MCQ
10 Questions MCQ Test Daily Test for JEE Preparation - Test: Stoichiometry and Stoichiometric Calculations (April 8)
In a reaction container, 100 g of hydrogen and 100 g of CI2 are mixed for the formation of HCl gas. What is the limiting reagent and how much HCl is formed in the reaction?
If 40 g of CaCO3 is treated with 40 g of HCl, which of the reactants will act as limiting reagent?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
How much oxygen is required for complete combustion of 560 g of ethene?
How much mass of sodium acetate is required to make 250 mL of 0.575 molar aqueous solution?
A solution is prepared by adding 5 g of a solute 'X' to 45 g of solvent 'Y'. What is the mass per cent of the solute 'X'?
2.82 g of glucose is dissolved in 30 g of water. The mole fraction of glucose in the solution is
The final molarity of a solution made by mixing 50 mL of 0.5 M HCl, 150 mL of 0.25 M HCl and water to make the volume 250 mL is
What is the concentration of copper sulphate (in mol L-1) if 80 g of it is dissolved in enough water to make a final volume of 3 L?
What volume of 5 M Na2SO4 must be added to 25 mL of 1 M BaCl2 to produce 10 g of BaSO4?
What will be the molality of the solution made by dissolving 10 g of NaOH in 100 g of water?
|
360 tests
|