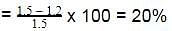Test: Thermochemistry (June 13) - JEE MCQ
10 Questions MCQ Test Daily Test for JEE Preparation - Test: Thermochemistry (June 13)
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
How much heat will be required at constant pressure to form 1.28 kg of CaC2 from CaO(s) & C(s) ?
Given :
ΔfH°(CaO, s) = -152 kcal/mol
ΔfH°(CaC2, s) = -14 kcal/mol
ΔfH°(CO, g) = -26 kcal/mol
50.0 mL of 0.10 M HCl is mixed with 50.0 mL of 0.10 M NaOH. The solution temperature rises by 3.0°C Calculate the enthalpy of neutralization per mole of HCl. [take proper assumptions]
The enthalpy of neutralisation of a weak acid in 1 M solution with a strong base is -56.1 kJ mol-1. If the enthalpy of ionization of the acid is 1.5 kJ mol-1 and enthalpy of neutralization of the strong acid with a strong base is -57.3 kJ equiv-1, what is % ionization of the weak acid in molar solution (assume the acid to be monobasic) ?
For the allotropic change represented by the equation C (graphite) → C (diamond), ΔH = 1.9 kJ. If 6 g of diamond and 6 g of graphite are separately burnt to yield CO2, the heat liberated in first case is
If x1, x2 and x3 are enthalpies of H - H, O = O and O - H bonds respectively, and x4 is the enthalpy of vaporisation of water, estimate the standard enthalpy of combustion of hydrogen
NH3(g) + 3Cl2(g) NCl3(g) + 3HCl(g) ; -ΔH1
N2(g) + 3H2(g) 2NH3(g) ; ΔH2
H2(g) + Cl2(g) 2HCl(g) ; ΔH3
The heat of formation of NCl3 (g) in the terms of ΔH1, ΔH2 and ΔH3 is
The enthalpy of neutralisation of HCl and NaOH is -57 kJ mol-1. The heat evolved at constant pressure (in kJ) when 0.5 mole of H2SO4 react with 0.75 mole of NaOH is equal to
Reaction involving gold have been of particular interest to a chemist. Consider the following reactions.
Au(OH)3 + 4 HCl → HAuCl4 + 3H2O, ΔH = -28 kcal
Au(OH)3 + 4 HBr → HAuBr4 + 3 H2O, ΔH = -36.8 kcal
In an experiment there was an absorption of 0.44 kcal when one mole of HAuBr4 was mixed with 4 moles of HCl. What is the percentage conversion of HAuBr4 into HAuCl4 ?
|
360 tests
|