Test: Reactions of Aldehydes & Ketones(9 Dec) - JEE MCQ
10 Questions MCQ Test Daily Test for JEE Preparation - Test: Reactions of Aldehydes & Ketones(9 Dec)
Only One Option Correct Type
Direction (Q, Nos. 1-9) This section contains 9 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
Which of the following will give a racemic mixture on reduction with NaBH4 followed by acid work-up?
What would be the major product in the following reaction?
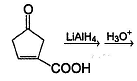
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following on reaction with excess of NaHSO3 in aqueous solution will give mixture of salts which can be separated into two fractions by fractional crystallisation?
Which is the most suitable reagent for the following transformation?
Which is the most suitable reagent for the following transformation?
The reagent which can best bring about the following transformation is
The most probable product of the following reaction is
Consider the following reaction,
Q.
The most likely organic product X is
Consider the following reaction,
Q.
The most likely organic product X is
One or More than One Options Correct Type
Direction (Q. Nos. 10-13) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Consider the following reaction,
Q.
The organic product(s) formed above is/are
|
360 tests
|


















