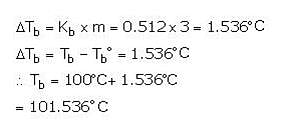Test: Ideal and non ideal solutions & Colligative properties(19 Sep) - JEE MCQ
10 Questions MCQ Test Daily Test for JEE Preparation - Test: Ideal and non ideal solutions & Colligative properties(19 Sep)
For an ideal solution with pA > pB, which of the following is true?
Intermolecular forces between n-hexane and n-heptane are nearly same as between hexane and heptane individually. When these two are mixed, which of the following is not true about the solution formed?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Study the figures given below and mark the correct statement.
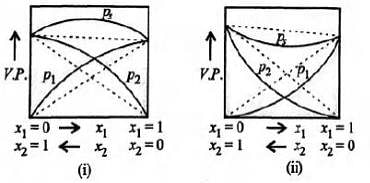

Given below are few mixtures formed by mixing two components. Which of the following binary mixtures will have same composition in liquid and vapour phase?
(i) Ethanol + Chloroform
(ii) Nitric acid + Water
(iii) Benzene + Toluene
(iv) Ethyl chloride + Ethyl bromide
The molal elevation constant is the elevation in boiling point of
What is the boiling point of a 3 molal aqueous solution if Kb is 0.512°C/m?
A solution was made by dissolving 2 g of a solute in 100 g of acetone. The solution boiled at 56.95° C. The boiling point of pure acetone is 55.95° C, and the Kb =1.71°C/m. What is the molecular weight of the solute?
Which of the two has lower freezing point, 2m NaCl or 5m NaCl aqueous solution?
Which of the following unit of concentration is independent of temperature?
Camphor is used as solvent to determine the molecular mass of non-volatile solute by Rast method because for camphor
|
360 tests
|