Test: Formal Charge & Resonance (May 27) - JEE MCQ
10 Questions MCQ Test Daily Test for JEE Preparation - Test: Formal Charge & Resonance (May 27)
Azide ion 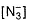 exhibits an (N—N) bond order of 2 and may be represented by resonance structures I, II and III given below :
exhibits an (N—N) bond order of 2 and may be represented by resonance structures I, II and III given below :

Select the correct statement(s) about more contributions,
C—Cl bond in 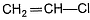 (vinyl chloride) is stabilised in the same way as in
(vinyl chloride) is stabilised in the same way as in
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
NO2 can be represented as 
Q. Formal charge on each oxygen atom is
Which representation for the Lewis structure of HNO3 is correct?
By SN 1 reaction allyl chloride changes to allyl alcohol as shown
Select the correct product isotope of carbon)
Stability of (C—Cl) bond in chlorobenzene is sim ilar to (C—Cl) bond in
Consider the following compounds
I. Vinyl chloride
II. B3N3H6
Delocalisation takes place
Hydrogen bonds are formed in many compounds e.g., H2O, HF, NH3. The boiling point of such compounds depends to a large extent on the strength of hydrogen bond and the number of hydrogen bonds. The correct decreasing order of the boiling points of above compounds is :
Out of the following two structures of N2O, which is more accurate?
Direction (Q. Nos. 16-17) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q. Select the correct statements about O3.
|
360 tests
|












