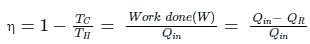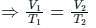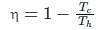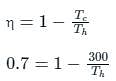Test: Basic Concepts of Thermodynamics & Applications (June 10) - JEE MCQ
10 Questions MCQ Test Daily Test for JEE Preparation - Test: Basic Concepts of Thermodynamics & Applications (June 10)
110 joule of heat is added to a gaseous system, whose internal energy is 40 J. Then the amount of external work done is
The heat given to an ideal gas in isothermal conditions is used to:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In thermodynamics ___________ is not a state variable.
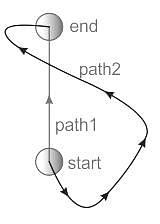
In which Thermodynamic process is there no flow of heat between the system and the surroundings?
If the temperature of the source is increased, the efficiency of the Carnot engine
Melting Point of pure ice is-
Which of the following is an intensive variable?
The system in which there is no exchange of matter, energy or work with the environment is called _________.
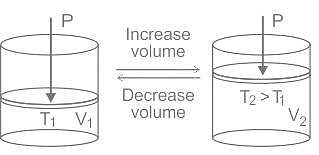
Which variable is held constant in Charles's Law?
What is the source temperature of the Carnot engine in K required to get 70% efficiency?
Given sink temperature = 27 °C
|
360 tests
|