Test: Bio Inorganic- 2 - Chemistry MCQ
30 Questions MCQ Test Inorganic Chemistry - Test: Bio Inorganic- 2
Oxidized form of enzyme Catalase (structure A); prepared by the reaction of [Fe(P)]+ (P = porphyrin) with H2O2 has a green color because of:
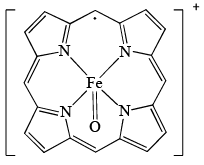
A (substituents on ring are removed for clarity)
At pH 7, the zinc(II) ion in carbonic anhydrase reacts with CO2 to give:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Molybdoenzymes can both oxidize as well as reduce the substrates, because:
Under physiological condition, oxygen is binding to deoxyhemoglobin and deoxymyoglobin, the binding curve and its pH dependence, respectively, are:
Match the metalloproteins in column A with their function in column B
The correct answer is
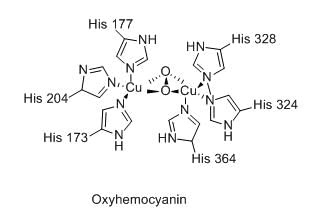
The total number of metal ion and the number of coordinated imidazole units of histidine in the active site of oxy-hemocyanin, respectively, are:
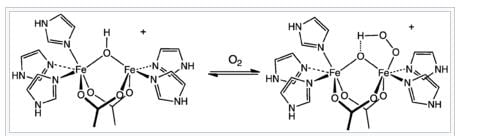
The changes (from A-D given below) which occur when O2 binds to hemerythrin are
(A) One iron atom is oxidized
(B) Both the iron atoms are oxidized
(C) O2 binds to one iron atom and is also hydrogen-bonded.
(D) O2 binds to both the iron atoms and is also hydrogen-bonded.
The metal present at the active site of the protein Carboxypept idase A is:
Match the following items of column I with the appropriate items in column II
In biological systems, the metal ion involved in the dioxygen transport besides Fe is:
Iron–sulphur clusters in bio logical systems are involved in:
The amino acid side chain high affinity for Ca2+ and Cu2+ in metallo–proteins is:
When a reduced cytochrome transfers an electron from its Fe(II) to the bound O2:
In photosynthesis, the predominant metal present in the reaction centre of photo –system II is:
Zn in carbonic anhydrase is coordinated by three histidine and one water molecule. The reaction of CO2 with this enzyme is an example of:
In biological systems, the metal ions involved in electron transport are:
In the transformation of oxyhaemoglobin to deoxyhaemoglobin:
Among the following pair of metal ions present in Nature. The first one functions as an electron transfer agent and the second one catalyzes the hydrolysis reactions. The correct pair is:
A well known naturally occurring organometallic compound is:
Hemoglobin is an oxygen carrying. The correct statement about oxy–hemoglobin is that:
Oxymyoglobin Mb(O2) and oxyhaemoglobin Hb(O2)4, respectively, are:
The number of oxygen molecule(s) that a molecule of hemerythrin can transport is:
Mg2+ is preferred in photosynthesis by chlorophyll because:
Among the given pH values, the O2 binding efficiency of hemoglobin is maximum at:
Identify the function of hemocyanin and the metal responsible for it:
During oxygen transport by hemerythrin, oxygen is bound as:
At pH 7.2 and 10 Torr oxygen partial pressure, the extent of O2 binding is:
|
48 videos|92 docs|41 tests
|