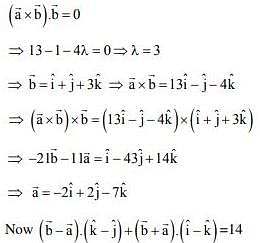MET Mock Test - 5 - JEE MCQ
30 Questions MCQ Test MET Mock Test Series - MET Mock Test - 5
The value of tension in a long thin metal wire has been changed from T1 to T2. The lengths of the metal wire at two different values of tension T1 and T2 are I1 and I2, respectively. The actual length of the metal wire is
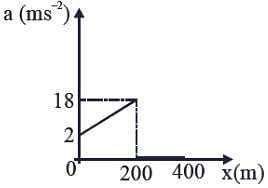
The velocity-displacement graph describing the motion of a bicycle is shown in the figure.
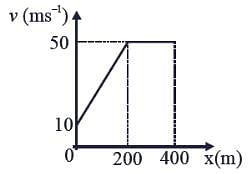
The acceleration-displacement graph of the bicycle's motion is best described by:

The acceleration-displacement graph of the bicycle's motion is best described by:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Two ideal Carnot engines operate in cascade (all heat given up by one engine is used by the other engine to produce work) between temperatures, T1 and T2. The temperature of the hot reservoir of the first engine is T1 and the temperature of the cold reservoir of the second engine is T2. T is temperature of the sink of first engine which is also the source for the second engine. How is T related to T1 and T2, if both the engines perform equal amount of work?
An electron (of mass m) and a photon have the same energy E in the range of a few eV. The ratio of de-Broglie wavelength associated with the electron and the wavelength of the photon is:
(c = speed of light in vacuum)
A particle is projected with a velocity v, so that its range on a horizontal plane is twice the greatest height attained. If g is acceleration due to gravity, then its range is
If the magnetic field in a plane electromagnetic wave is given by  = 3 × 10-8 sin (1.6 × 103x + 48 × 1010t)
= 3 × 10-8 sin (1.6 × 103x + 48 × 1010t)  T, then what will be the expression for electric field?
T, then what will be the expression for electric field?
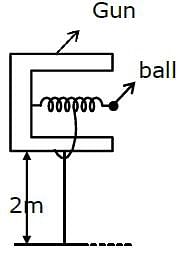
In a spring gun having spring constant 100 N/m, a small ball 'B' of mass 100 g is put in its barrel (as shown in figure) by compressing the spring through 0.05 m. There should be a box placed at a distance 'd' on the ground so that the ball falls in it. If the ball leaves the gun horizontally at a height of 2 m above the ground, the value of d is _______________ m. (g = 10 m/s2)
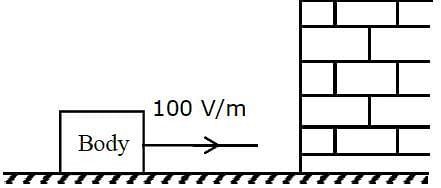
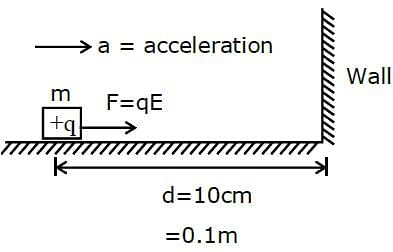
A body having specific charge 8 μC/g is resting on a frictionless plane at a distance 10 cm from the wall (as shown in the figure). It starts moving towards the wall when a uniform electric field of 100 V/m is applied horizontally towards the wall. If the collision of the body with the wall is perfectly elastic, then the time period of the motion will be ______________ s.
The Ksp for bismuth sulphide (Bi2S3) is 1.08 × 10-73. The solubility of Bi2S3 in mol L-1 at 298 K is
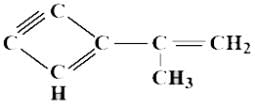
In the analysis of an organic compound, it was found that it contains 7 carbon atoms, there are two C = C bonds and one C ≡ C bond. This compound is a hydrocarbon.
Hydrocarbons have only carbon and hydrogen elements. On structural analysis it was found that it is covalent in nature and expected structure is given above. The ratio between the pure and hybrid orbitals is?
Which one of the following compounds is used as a chemical in certain type of fire extinguishers?
Given below are two statements.
Statement I: The presence of weaker π-bonds make alkenes less stable than alkanes.
Statement II: The strength of the double bond is greater than that of carbon-carbon single bond.
In the light of the above statements, choose the correct answer from the options given below.
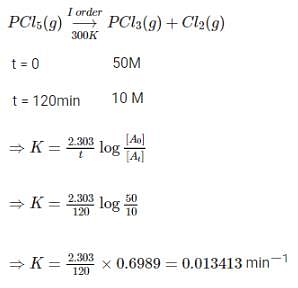
PCl5(g) → PCl3(g) + Cl2(g)
In the above first order reaction, the concentration of PCl5 reduces from initial concentration 50 mol L-1 to 10 mol L-1 in 120 minutes at 300 K. The rate constant for the reaction at 300 K is X × 10-2 min-1. The value of x is ___________.(Nearest integer)
[Given log 5 = 0.6989]
The concentration of dissolved oxygen (DO) in cold water can go upto: (Answer up to the nearest integer)
Let A and B are two events and P(A′) = 0⋅3, P(B) = 0⋅4, P(A∩B′) = 0⋅5 then P(A∪B′) is:
Directions: The following question has four choices, out of which ONLY ONE is correct.
Find the approximate value of where [ ] denotes g.i.f.
If ω ≠ 1 is a cube root of unity and satisfies and
then the value of
is
In a sequence of number P, Q, R, S, T, U, V, ..., Z, the value of each subsequent number is 3 more than the product of previous and 3. If it is known that T is equal to 21, what is the value of P + Q?
If e and e′ are the respective eccentricities of hyperbolas  , then
, then
If the sum of the squares of the intercepts on the axes cut off by the tangent to the curve x1/3 + y1/3 = a1/3 (a > 0) at (a/8, a/8) is 2, then the value of 'a' is
Let A = {aii} be a 3 × 3 matrix, where
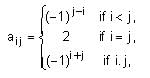
then det(3 adj(2A-1)) is equal to ________.
Improve the sentence by choosing best alternative for capitalised part of the sentence.
After a few minutes the unconscious boxer began to come OUT.
|
10 tests
|


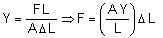
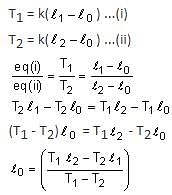
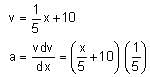
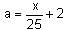
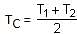
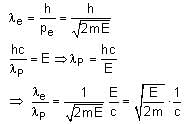
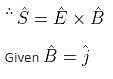
 V/m
V/m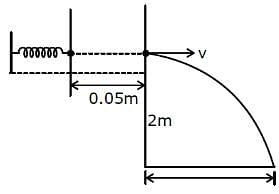
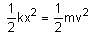
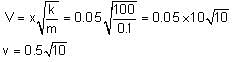
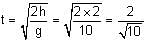
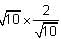
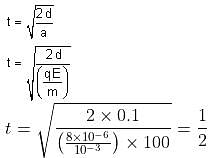
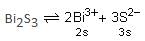
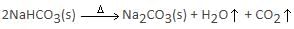
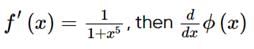 is equal to
is equal to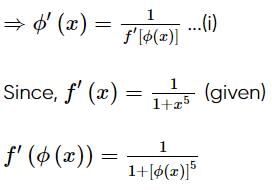
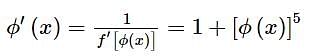
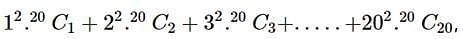 given
given 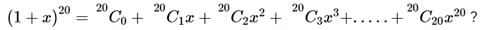
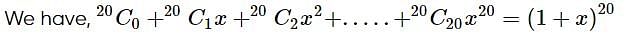
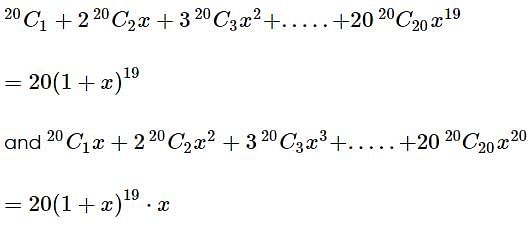
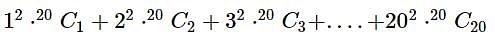
 , then UV + XY
, then UV + XY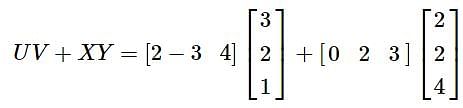
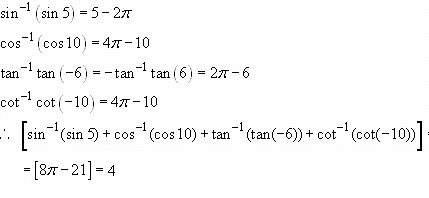
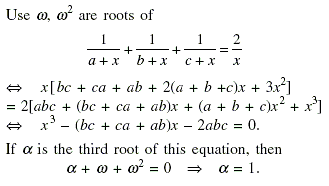

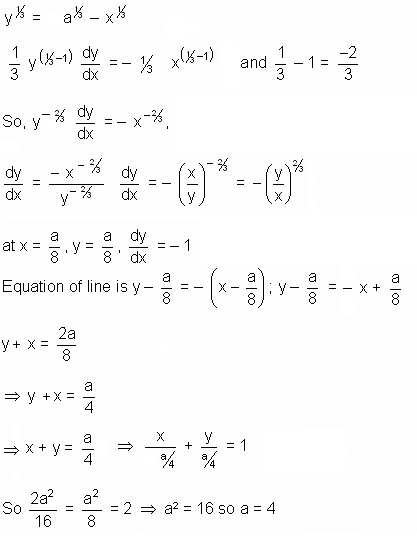
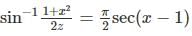 is/are
is/are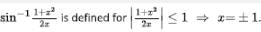 Out of these two values of x, on;y x=1 satisfies the given equation
Out of these two values of x, on;y x=1 satisfies the given equation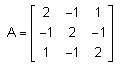
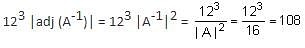
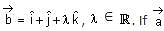 is a vector such that
is a vector such that 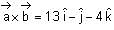 and
and 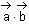 + 21 = 0, then
+ 21 = 0, then 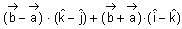 is equal to
is equal to