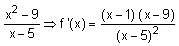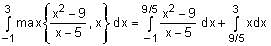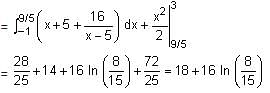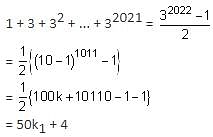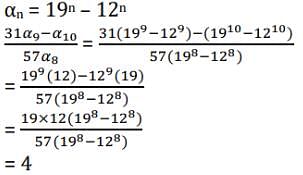MET Mock Test - 9 - JEE MCQ
30 Questions MCQ Test MET Mock Test Series - MET Mock Test - 9
If the charge on a capacitor is increased by 2 C, the energy stored in it increases by 44%. The original charge on the capacitor is (in C)
Two identical charged particles each having a mass 10 g and charge 2.0 x 10-7 C are placed on a horizontal table with a separation of L between them such that they stay in limited equilibrium. If the coefficient of friction between each particle and the table is 0.25, find the value of L. [Use g = 10 ms-2]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
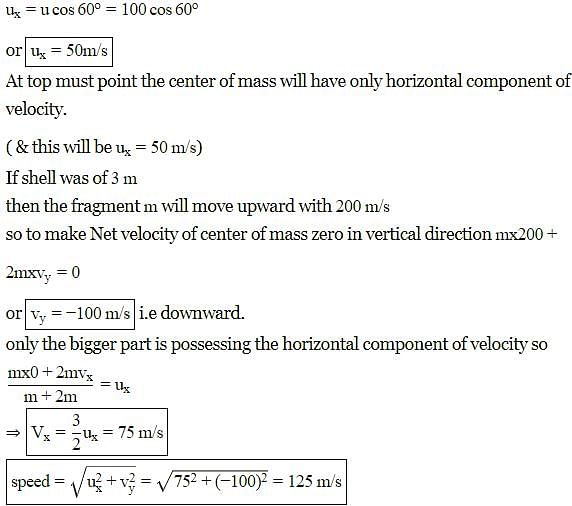
A shell is fired from a cannon with a speed of 100 ms-1 at an angle 30° with the vertical (y-direction). At the highest point of its trajectory, the shell explodes into two fragments of masses in the ratio 1 : 2. The lighter fragment moves vertically upwards with an initial speed of 200 ms-1. What is the speed of the heavier fragment at the time of explosion?
An iron wire AB of length 3 m at 0°C is stretched between the opposite walls of a brass casing at 0°C0°C as shown in the figure below. The diameter of the wire is m0.6 mm. What extra tension will be set up in the wire when the temperature of the system is raised to 40°C?
Given αbrass = 18 × 10−6 K−1, αiron= 12 × 10−6 K−1, Yiron = 21 × 1010 N m−2
Two satellites S1 and S2 are revolving in circular orbits around a planet with radius R1 = 3200 km and R2 = 800 km, respectively. The ratio of speed of satellite S1 to the speed of satellite S2 in their respective orbits would be 1/x where x =
When light of frequency twice the threshold frequency is incident on the metal plate, the maximum velocity of emitted electron is v1. When the frequency of incident radiation is increased to five times the threshold value, the maximum velocity of emitted electron becomes v2. If v2 = xv1, the value of x will be ________. (In Integers)
The highest industrial consumption of molecular hydrogen is to produce compounds of element:
The predominant intermolecular forces present in ethyl acetate, a liquid, are:
The correct order of electron gain enthalpies of Cl, F, Te and Po is
Metals generally melt at very high temperature. Amongst the following, the metal with the highest melting point will be
Cerium (IV) has a noble gas configuration. Which of the following is correct statement about it?
Manganese (VI) has ability to disproportionate in acidic solution. The difference in oxidation states of two ions it forms in acidic solution is _____. (In integers)
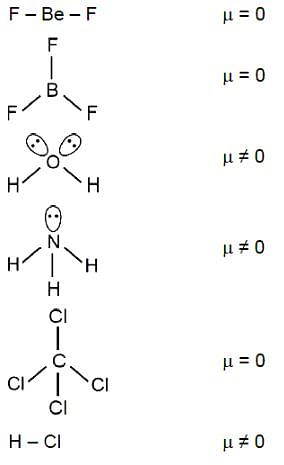
Amongst BeF2, BF3, H2O, NH3 CCl4 and HCl, the number of molecules with non-zero net dipole moment is _________.
The standard entropy change for the reaction
4Fe(s) + 3O2(g) → 2Fe2O3(s) is -550 JK-1 at 298 K.
[Given: The standard enthalpy change for the reaction is -165 kJ mol-1]. The temperature in K at which the reaction attains equilibrium is __________. (Nearest Integer)
A rigid nitrogen tank stored inside a laboratory has a pressure of 30 atm at 06:00 am when the temperature is 27°C. At 03:00 pm, when the temperature is 45°C, the pressure in the tank will be _______ atm. [nearest integer]
Let f(x) = xcos-1(-sin|x|), x ∈ 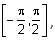 then which of the following is true?
then which of the following is true?
The number of values of α for which the system of equations
x + y + z = α
αx + 2αy + 3z = -1
x + 3αy + 5z = 4
is inconsistent, is
If the sum of the squares of the reciprocals of the roots α and β of the equation 3x2 + λx - 1 = 0 is 15, then 6(α3 + β3)2 is equal to:
Let Q be the mirror image of the point P(1, 0, 1) with respect to the plane S: x + y + z = 5. If a line L passing through (1, -1, -1), parallel to the line PQ meets the plane S at R, then QR2 is equal to:
The remainder on dividing 1 + 3 + 32 + 33 + ... + 32021 by 50 is _________. (In integers)
For a natural number n, let an = 19n - 12n. Then, the value of 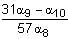 is
is
Out of the given alternatives, choose the one which can be substituted for the given capitalised word.
The old man CUT TO THE QUICK when his rich son refused to recognise him.
Improve the sentence by choosing best alternative for capitalised part of the sentence.
After a few minutes the unconscious boxer began to come OUT.
|
10 tests
|


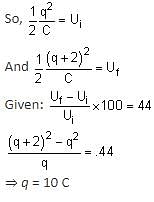

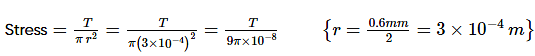
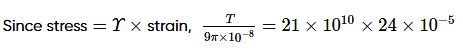
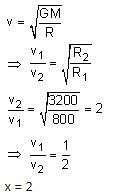
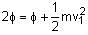 ___ (1) and
___ (1) and 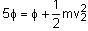 ____ (2)
____ (2)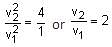
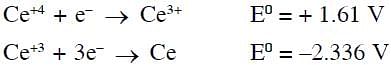
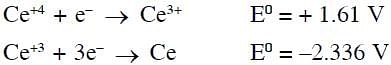
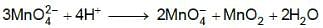
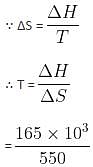
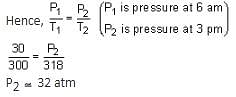
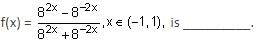
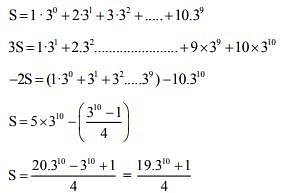
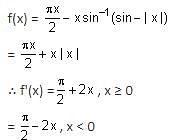
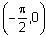 and increasing in
and increasing in 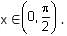
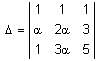
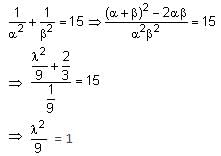
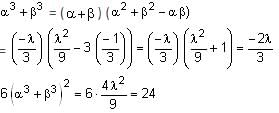
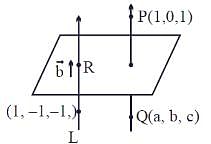
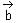 mirror image of Q on given plane x + y + z = 5
mirror image of Q on given plane x + y + z = 5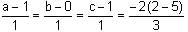
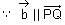
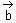 = (1, 1, 1)
= (1, 1, 1)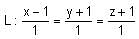
 . If
. If  , then T + n(S) is equal to:
, then T + n(S) is equal to:

 .
.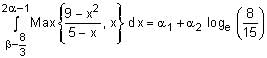 , then α1 + α2 is equal to ________. (in integer)
, then α1 + α2 is equal to ________. (in integer)