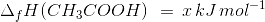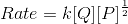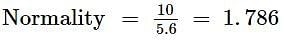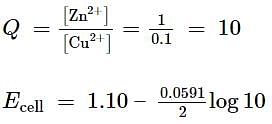COMEDK Mock Test - 5 - JEE MCQ
30 Questions MCQ Test COMEDK Mock Test Series - COMEDK Mock Test - 5
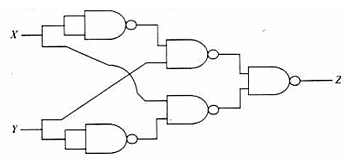
The logic gate circuit shown in the figure realises which of the following functions?

Population of a town is reported as 157,900 . Which of the following statements correct?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The de-Broglie wavelength associated with an electron and a proton were calculated by accelerating them through same potential of 100 V. What should nearly be the ratio of their wavelengths? (mp = 1.00727 u, me = 0.00055 u)
Three particles P, Q and R placed as per given figure. Masses of P, Q and R are √3 m, √3 m and m respectively. The gravitational force on a fourth particle ‘S’ of mass m is equal to
Let nr and nb be respectively the number of photons emitted by a red bulb and a blue bulb of equal power in a given time.
A particle moving along x-axis has acceleration 'f' at time 't', given by ![]() , where 'f0' and 'T' are constants. The particle at 't' = 0 has zero velocity. In the time interval between 't' = 0 and the instant when 'f' = 0, the velocity (vx) of the particle is
, where 'f0' and 'T' are constants. The particle at 't' = 0 has zero velocity. In the time interval between 't' = 0 and the instant when 'f' = 0, the velocity (vx) of the particle is
Two soap bubbles with radii come in contact. Their common surface has a radius of curvature r. Then
If force 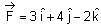 acts on a particle having position vector
acts on a particle having position vector 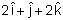 , then the torque about the origin will be:
, then the torque about the origin will be:
Two light beams of intensities in the ratio of 9 : 4 are allowed to interfere. The ratio of the intensity of maxima and minima will be:
The length, breadth and thickness of a rectangular sheet of metal are 4.234 m, 1.005 m, and 2.01 cm respectively. Give the volume of the sheet to correct significant figures.
Among the lanthanide Eu, Tb, Er and Dy, which one readily forms stable divalent ions?
If the ionisation potential of hydrogen atom is 2 x 10-18 J, then the frequency of Hβ line of Balmer series is
(Given: Plank's constant = 6.6 x 10-34 Js)
For the reaction 2H2 + O2 2H2O, H = -571 kJ, bond energy of H - H = 435 kJ/mol and that of O - O = 498 kJ/mol. Calculate the average bond energy (in kJ/mol) of O - H bond using this data.
Excess of iron is added to a 0.1 M Cd2+ solution and the system is allowed to attain equilibrium.
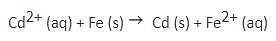
The concentration of Cd2+ ions, if Eo = 0.037 V, is
Directions: The following question has four choices out of which ONLY ONE is correct.
The heat of neutralisation of acetic acid by sodium hydroxide is −55.9 kJ/mol, and the heat of neutralisation of a strong acid by a strong base is −57.1 kJ/mol. What is the value of ΔH for the ionisation of CH3COOH?
Consider the reaction:
P + 2Q → R
When concentration of Q alone was tripled, the half-life did not change. When the concentration of P alone was made four times, the rate increased two times. The unit of rate constant for this reaction would be
A solution has a 1 : 4 mole ratio of pentane to hexane. The vapour pressures of the pure hydrocarbons at 20oC are 440 mm of Hg for pentane, and 120 mm of Hg for hexane. The mole fraction of pentane in the vapour phase would be
If 10 volume of H2O2 reacts completely with 100 ml of 2 N KMnO4, calculate the volume of H2O2 used in mL.
For the redox reaction,

taking place in a cell, E∘cell is 1.10 V. E∘cell for the cell will be

The equation of the parabola whose focus is (1,-1)and directrix is x + y + 7 = 0 is
Let A = { x ∈ R : x ≠ 0, − 4 ≤ x ≤ 4 } and f : A → R is defined by f (x) = | x |/x for x ∈ A . Then the range of f is
The condition for the roots of the equation (c2-ab)x2-2(a2-bc)x+(b2-ac)=d are equal, is
|
10 tests
|





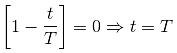

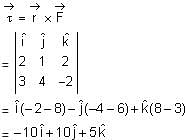
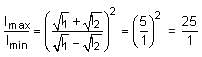







 for a strong acid and a strong base =
for a strong acid and a strong base =