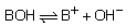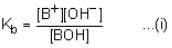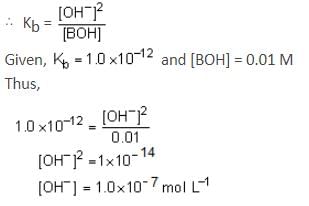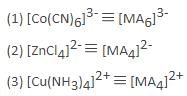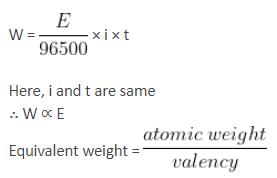COMEDK Mock Test - 9 - JEE MCQ
30 Questions MCQ Test - COMEDK Mock Test - 9
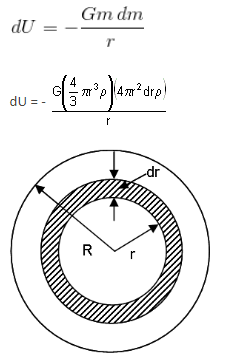
Consider a solid sphere of mass M and radius R. The energy released in forming this solid sphere is
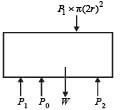
A cylindrical tank has a hole of diameter 2r in its bottom. The hole is covered wooden cylindrical block of diameter 4r, height h and density ρ/3.
Situation I : Initially, the tank is filled with water of density ρ to a height such that the height of water above the top of the block is h1 (measured from the top of the block).
Situation II : The water is removed from the tank to a height h2 (measured from the bottom of the block), as shown in the figure.
The height h2 is smaller than h (height of the block) and thus the block is exposed to the atmosphere.
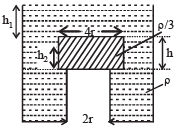
Q. Find the minimum value of height h1 (in situation 1), for which the block just starts to move up?
The height h2 is smaller than h (height of the block) and thus the block is exposed to the atmosphere.

During the magnetic braking of trains if the north and the south poles are replaced with each other, then the velocity of the train will:
If force F is related with distance x and time t as F = A√x + Bt2 , the dimensions of A/B is
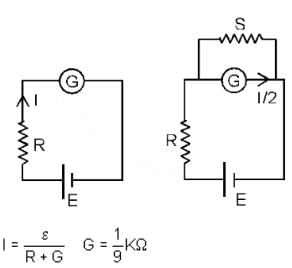
In a circuit for finding the resistance of a galvanometer by half deflection method, a 6 V battery and a high resistance of 11 kΩ are used. The figure of merit of the galvanometer is 60 μA/division. In the absence of shunt resistance, the galvanometer produces a deflection of θ = 9 divisions, when current flows in the circuit. The value of the shunt resistance that can cause the deflection of θ/2 is closest to:
A 25 m long antenna is mounted on an antenna tower. The height of the antenna tower is 75 m. The wavelength (in metres) of the signal transmitted by this antenna would be:
When a system is taken from state i to state f along the path iaf, it is found that Q = 50 cal and W = 20 cal. Along the path ibf, Q = 36 cal. W along the path ibf is
Which of the following ions having following electronic structure would have the maximum magnetic moment?
At 25°C, the dissociation constant of a base, BOH, is 1.0 x 10-12. The concentration of hydroxyl ions in 0.01 M aqueous solution of the base would be
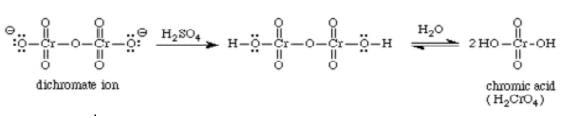
Which of the following is the true covalent oxide of iodine?
If the aqueous solutions of the following salts are electrolysed for 1 h with 10 A current, then which of the following solutions will deposit the maximum mass of the metal at the cathode?
(The atomic weights are Fe = 56 u, Zn = 65 u, Ag = 108 u, Hf = 178 u and W = 184 u)
If z and ω are two non-zero complex numbers such that |z ω | = 1, and arg(z) - arg(ω)= π/2,then z̅ω is equal to
The differential equation which represents the family of plane curves y=exp. (cx) is
If f(x) = cos (logx), then f(x) f(y) -1/2[f(x/y) + f(xy)] has the value
If the value of a determinants is 11, then the square of the determinant formed by its cofactor will be
If m1, m2 are slopes of the two tangents that are drawn from (2, 3) to the parabola y2 = 4x, then the value of [(1/m1) + (1/m2)] is
The sides of an equilateral triangle are increasing at the rate of 2 cm/sec. The rate at which the area increases, when the side is 10 cm is
The differential equation of all circles which pass through (0,0) and having centre on y-axis, is


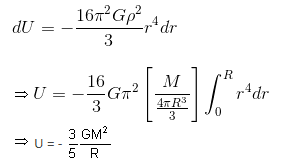
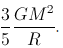
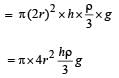
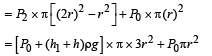
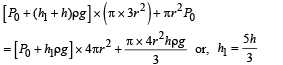

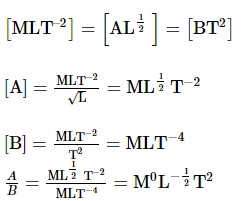
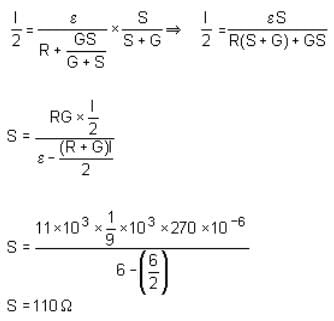

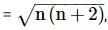 where n is the number of unpaired electrons.
where n is the number of unpaired electrons.