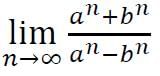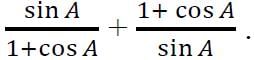Biotechnology - 2019 Past Year Paper - IIT JAM MCQ
30 Questions MCQ Test IIT JAM Past Year Papers and Model Test Paper (All Branches) - Biotechnology - 2019 Past Year Paper
The glycosidic linkages in cellulose and amylose are ________, respectively.
A mutation in the operator locus of lac operon that confers constitutive expression of β-galactosidase is ________.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which one of the points 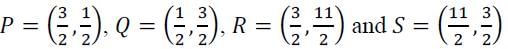 lies ABOVE the parabola y=2x2 and INSIDE the circle x2+y2 =4 ?
lies ABOVE the parabola y=2x2 and INSIDE the circle x2+y2 =4 ?
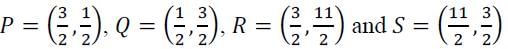 lies ABOVE the parabola y=2x2 and INSIDE the circle x2+y2 =4 ?
lies ABOVE the parabola y=2x2 and INSIDE the circle x2+y2 =4 ?Let U= {1, 2, 3, 4, 5.} A subset S is chosen uniformly at random from the non-empty subsets of U. What is the probability that S does NOT have two consecutive elements?
Which one of the following figures represents the correct sequence of phases in adult eukaryotic cell cycle?
At what pH does poly-Glu in an aqueous solution form α-helical structure?
The dimensions of coefficient of viscosity are _______.
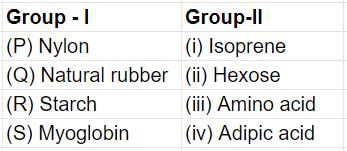
Match the entries in Group I with the entries in Group II
The technique that involves impacting samples with electrons is _______.
The orbital angular momentum of hydrogen atom in the ground state is ________.
In how many ways can one write the elements 1, 2, 3, 4 in a sequence 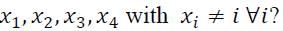
The evolution of eyes in octopus and in human is an example of ________.
Which one of the following modifications occurs both on DNA and protein?
Solutions of the following peptides are prepared separately at a concentration of 1 mM. Amongthese four, which one has the highest A280?
The free energy required to synthesize a mixed anhydride bond of 1,3-bisphosphoglycerate isgenerated by the oxidation of ________.
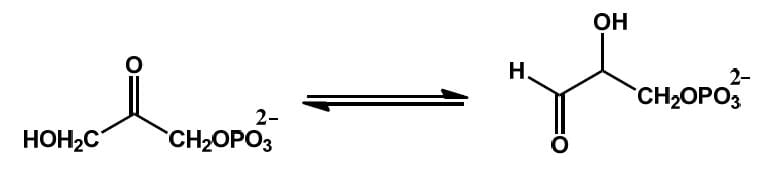
The following reaction is an example of ___________.
Which one of the following parameters changes upon doubling the enzyme concentration?
Which one of the following statements is a correct description of modes of action of taxol andcolchicine?
Which one of the following statements is INCORRECT with respect to bacterial conjugation?
A particle starting from rest is subjected to a constant force. The plot of distance traveled along thedirection of the force as a function of time is a/an ______.
Indole acetic acid (IAA) is involved in ______.
Which one of the following remains unchanged when light waves enter water from air?
According to the kinetic theory of gases, the average energy of a diatomic molecule in an ideal gasdepends on _______.
Match the entries in Group I with entries in Group II

H2 reacts with trans-(Ph3P)2Ir(CO)Cl to primarily produce _______.
Among the following species, the metal center that has the highest number of unpaired electrons is _______.
|
29 docs|48 tests
|
|
29 docs|48 tests
|


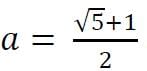 and
and 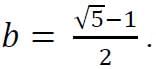 Then,
Then,