Chemistry - 2016 Past Year Paper - IIT JAM MCQ
30 Questions MCQ Test IIT JAM Past Year Papers and Model Test Paper (All Branches) - Chemistry - 2016 Past Year Paper
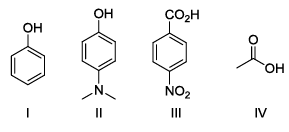
The correct order of pKa for the following compounds is
The major product formed in the following reaction is
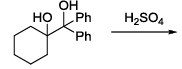
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The mechanism of the following transformation involves

The crystal field stabilization energy (CFSE) in [Mn(H2O)6]2+ is
Among the following, the compound that has the lowest degree of ionic character is
The correct order of entropy for various states of CO2 is
The coordination numbers of Cs+ and Cl– ions in the CsCl structure, respectively, are
The correct order of 1H NMR chemical shiftvalues for the labeled methyl groups in the following compound is
Among the following, the most stable conformation of meso-2,3-dibromobutane is
The major products X and Y in the following reaction sequence are
The major product formed in the reaction of butanenitrile with phenylmagnesium bromide followed by acidification is
An organic compound on reaction with 2,4-dinitrophenylhydrazine (2,4-DNP) gives a yellow precipitate. It also gives silver mirror on reaction with ammoniacal AgNO3. It gives an alcohol and sodium salt of a carboxylic acid on reaction with concentrated NaOH. It yields benzene-1,2-dicarboxylic acid on heating with alkaline KMnO4. The structure of the compound among the following is
The major products X and Y in the following reaction sequence are
The complexes [Pt(CN)4]2– and [NiCl4]2–, respectively, are
The value of ‘x’ in [Cu(CO)x]+ such that it obeys the 18 electron rule is
The correct order of νNO (cm–1) in the following compounds is
The final products in the reaction of BF3 with water are
The correct order of bond angles in BF3, NH3, NF3 and PH3 is
At 298 K, 0.1 mol of ammonium acetate and 0.14 mol of acetic acid are dissolved in 1 L of water. The pH of the resulting solution is
[Given: pKa of acetic acid is 4.75]
An electrochemical cell consists of two half-cell reactions
AgCl(s) + e– → Ag(s) + Cl–(aq)
Cu(s) → Cu2+(aq) + 2e–
The mass of copper (in grams) dissolved on passing 0.5 A current for 1 hour is
[Given: atomic mass of Cu is 63.6; F = 96500 C mol–1]
For a zero order reaction, the half-life depends on the initial concentration [C0] of the reactant as
The effective nuclear charge of helium atom is 1.7. The first ionization energy of helium atom in eV is
The relationship between the van der Waals ‘b’ coefficient of N2 and O2 is
From the kinetic theory of gases, the ratio of most probable speed (Cmp) to root mean square speed (Crms) is
|
29 docs|48 tests
|
|
29 docs|48 tests
|

















