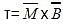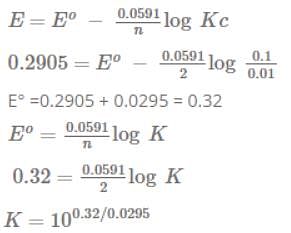Practice Test - NEET MCQ
30 Questions MCQ Test 4 Months Preparation for NEET - Practice Test
Which of the following statements about earth's magnetism is correct
A toroid wound with 60.0 turns/m of wire carries a current of 5.00 A. The torus is iron, which has a magnetic permeability of μm=5000μ0 under the given conditions. H and B inside the iron are
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
A bar magnet of magnetic moment 1.5 J/T lies aligned with the direction of a uniform magnetic field of 0.22 T. What is the amount of work required by an external torque to turn the magnet so as to align its magnetic moment opposite to the field direction?
In the magnetic meridian of a certain place, the horizontal component of the earth’s magnetic field is 0.26G and the dip angle is 60o. What is the magnetic field of the earth at this location
A bar magnet of magnetic moment 1.5 J/T lies perpendicular to the direction of a uniform magnetic field of 0.22 T. What is the torque acting on it?
Which of the following conditions are satisfied when the cell reaction in the electrochemical cell is spontaneous?
What is the observation when the opposing external applied potential to an electrochemical cell is greater than the cell’s potential?
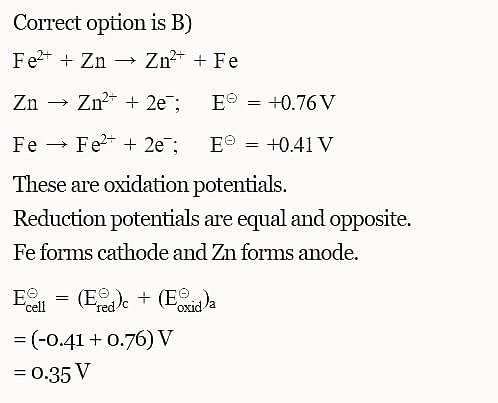
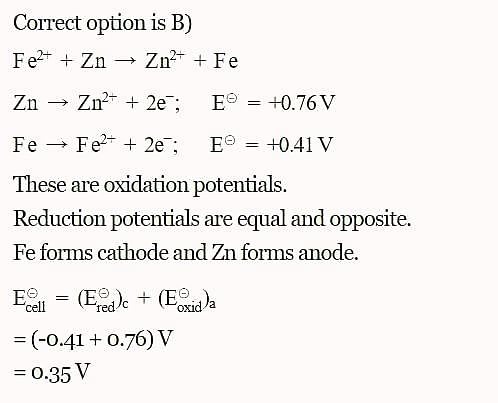
The standard reduction potentials E°, for the half reactions are as:
The emf for the cell reaction,
The correct order of equivalent conductance at infinite dilution of LiCl, NaCl and KCl is:
The specific conductances of four electrolytes in ohm−1cm−1 are given below. Which one offers higher resistance to passage of electric current?
The emf of the above cell is 0.2905 V. Equilibrium constant for the cell reaction is:
Given, standard electrode potentials, Fe2+ + 2e- →Fe, E° = -0.440V
Fe3+ +3e- → Fe, E° = -0.036V
The standard electrode potential (E°) for Fe3+ + e- → Fe2+ is:
In agarose gel electrophoresis, DNA molecules are separated on the basis of their:
Origin of replication is the specific DNA sequence on chromosome that which is responsible for:
The group of letters that form same words when read both forward and backward are called?
Read the given statements and select the correct options
Statement 1: In recombinant DNA technology, human genes are often transferred into bacteria (prokaryotes) or yeast (eukaryote).
Statement 2: Both bacteria and yeast multiply very fast to form huge population which expresses the desired gene.
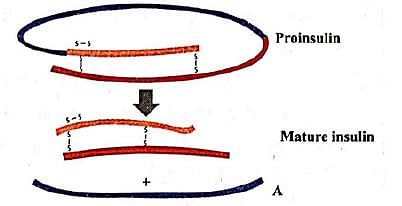
Given figure represents the maturation of pro-insulin into insulin. Identify the product A.
What might be an advantage of beginning gene therapy prior to birth?
|
514 videos|1455 docs|646 tests
|
|
514 videos|1455 docs|646 tests
|