Test: Geometrical Isomerism - 1 - NEET MCQ
21 Questions MCQ Test Chemistry Class 11 - Test: Geometrical Isomerism - 1
Direction (Q. Nos. 1-13) This section contains 13 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q.
Which among the following defines Meso forms of isomers
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Which among the following defines Meso forms of isomers
Which of the following compounds have Z-configuration?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In which type of projection we can get staggered and eclipsed conformations?
A cyclic dichloride has a total five constitutional plus geometrical isomers. Which of the following satisfy this condition without altering the carbon skeleton?
Which of the following carbonyls has four different enol isomers?
How many different stereoisomers exist for 1-chloro-2-(3-chlorocyclobutyl) ethene?
Which of the following has three different stereoisomers?
Which among the following does not exhibit geometric isomerism
'Which carbonyls below, on protonation gives a pair of stereoisomers?
A stereoisomer of cyclobutane-1,2-diol has lower solubility in water than its other stereoisomer. Which is this isomer and why?
How many cyclic isomers exists (structural and geometrical only) for C3H3CI3?
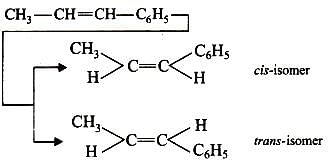
which compound below can show geometrical isomerism ?
Direction (Q. Nos. 14-18) This section contains 5 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q.
Which compound below can have a non-polar steroisomers ?
Which of the following is/are expected to form significant intramolecular H-bond?
Choose the correct statement(s) regarding geometrical isomerism?
Which of the following compounds will show geometrical isomerism?
[IIT JEE 1998]
The correct statements(s) concerning the structures E,F and G is (are)
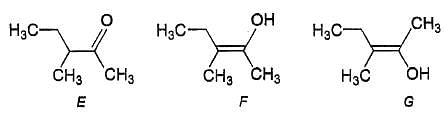
[IIT JEE 2008]
Direction (Q. Nos. 19-22) This sectionis based on statement I and Statement II. Select the correct answer from the code given below
Q.
Statement I Fluoroethanal has two stereomeric enols in which cis enol predominates at equilibrium.
Statement II Intramolecular H-bonding increases the stability of a stereomer.
Q.
Statement I : Butanone, upon protonation at oxygen atom result in a pair of stereoisomer.
Statement II : Presence of lone pair of electrons at oxygen is responsible for protonation.
Q.
Statement I : 1,2-dichlorocyclopropane has two stereoisomers.
Statement II : Restricted rotation is responsible for cis and trans-isomers of 1,2-dichlorocyclopropane.
Q.
Statement I : 1,2,3,4-tetrachloro-1,3-butadiene has two non-polar stereoisomers,
Statement II : Out of its four stereoisomers, two of them are polar.
|
127 videos|232 docs|88 tests
|


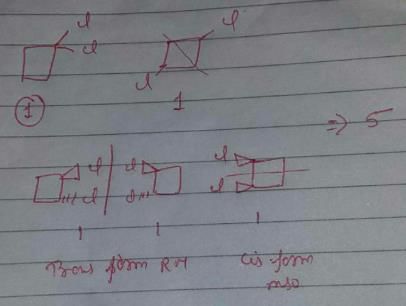 Only option b exists with 5 constitutional + geometrical isomers without altering carbon skeleton.
Only option b exists with 5 constitutional + geometrical isomers without altering carbon skeleton.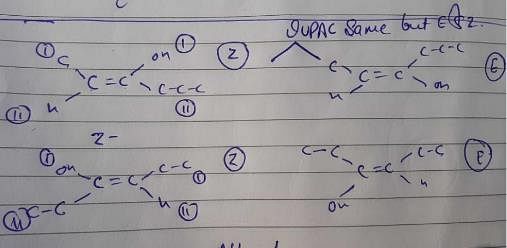
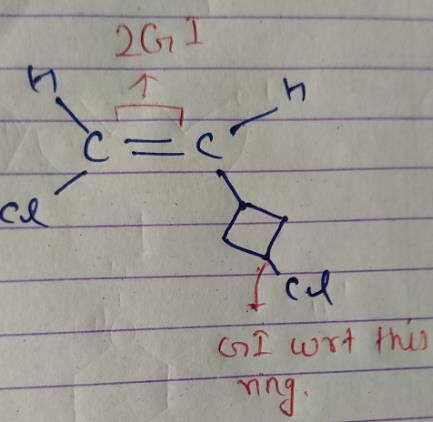
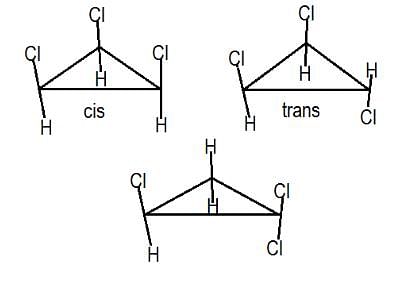

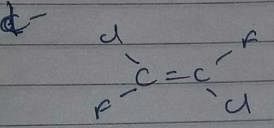
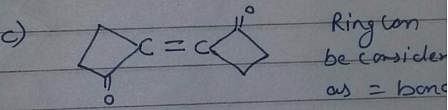 However, there is no such arrangement in structure i, it won’t have any non polar structure.
However, there is no such arrangement in structure i, it won’t have any non polar structure.