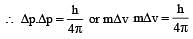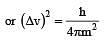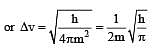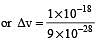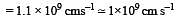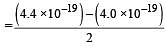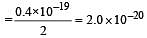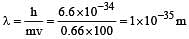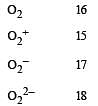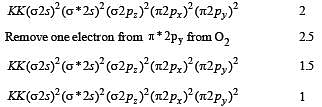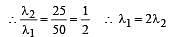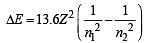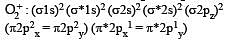31 Year NEET Previous Year Questions: Structure of Atom - 1 - NEET MCQ
30 Questions MCQ Test Chemistry Class 11 - 31 Year NEET Previous Year Questions: Structure of Atom - 1
The following quantum numbers are possible for how many orbital (s) n = 3, l = 2, m = +2 ? [2001]
Which of the following is isoelectronic? [2002]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In hydrogen atom, energy of first excited state is –3.4 eV. Find out KE of the same orbit of Hydrogen atom [2002]
The value of Planck's constant is 6.63 × 10–34 Js.The velocity of light is 3.0 × 108 m s–1. Which value is closest to the wavelength in nanometers of a quantum of light with frequency of 8 × 1015 s–1 ? [2003]
The ions O2–, F–, Na+, Mg2+ and Al3+ are isoelectronic. Their ionic radii show [2003]
The frequency of radiation emitted when the electron falls from n = 4 to n = 1 in a hydrogen atom will be (Given ionization energy of H=2.18 ×10–18J atom–1and h = 6.625 × 10–34 J s ) [2004]
The energy of the second Bohr orbit of the hydrogen atom is -328 kJ mol-1; hence the energy of the fourth Bohr orbit would be: [2005]
Given : The mass of electron is 9.11 × 10–31 kg Plank constant is 6.626 × 10–34 Js, the uncertainty involved in the measurement of velocity within a distance of 0.1 Å is [2006]
The orientation of an atomic orbital is governed by [2006]
Consider the following sets of quantum numbers:
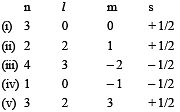
Which of the following sets of quantum number is not possible? [2007]
If uncertainty in position and momentum are equal, then uncertainty in velocity is : [2008]
The measurement of the electron position if associated with an uncertainty in momentum, which is equal to 1 × 10–18 g cm s– 1 . The uncertainty in electron velocity is? (mass of an electron is 9 × 10– 28 g)
[2008]
The energy absorbed by each molecule (A2) of a substance is 4.4 × 10–19 J and bond energy per molecule is 4.0 × 10–19 J. The kinetic energy of the molecule per atom will be: [2009]
Maximum number of electrons in a subshell of an atom is determined by the following: [2009]
Wh ich of the followin g is not per missible arrangement of electrons in an atom? [2009]
The electronic configuration for oxygen is written as 1s22s22p4. Which rule will this configuration be violating?
Which one of the following species does not exist under normal conditions? [2010]
A 0.66 kg ball is moving with a speed of 100 m/s.The associated wavelength will bexh (6.6x10-34 Js) : [2010]
The total number of atomic orbitals in fourth energy level of an atom is : [2011]
Which of the following has the minimum bond length ? [2011]
The energies E1 and E2 of two radiations are 25 eV and 50 eV, respectively. The relation between their wavelengths i.e., λ1 and λ2 will be :[2011]
If n = 6, the correct sequence for filling of electrons will be : [2011]
According to the Bohr Theory, which of the following transitions in the hydrogen atom will give rise to the least energetic photon ? [2011 M]
The pairs of species of oxygen and their magnetic behaviours are noted below. Which of the following presents the correct description? [2011 M]
Maximum number of electrons in a subshell with : l = 3 and n = 4 is : [2012]
The correct set of four quantum numbers for the valence electron of rubidium atom (Z = 37) is [2012]
The orbital angular momentum of a p-electron is given as : [2012 M]
The value of Planck’s constant is 6.63 × 10–34 Js.The speed of light is 3 × 1017 nm s–1.. Which value is closest to the wavelength in nanometer of a quantum of light with frequency of 6 × 1015 s–1? [NEET 2013]
What is the maximum numbers of electrons that can be associated with the following set of quantum numbers? n = 3, l = 1 and m = –1 [NEET 2013]
Based on equation 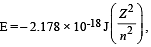 certain conclusions are written. Which of them is not correct ? [NEET 2013]
certain conclusions are written. Which of them is not correct ? [NEET 2013]
|
129 videos|233 docs|88 tests
|



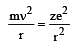
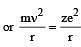
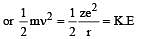
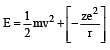
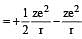
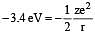
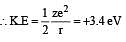
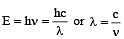
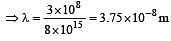
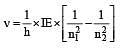
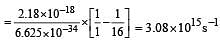
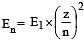
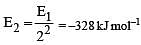
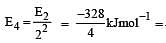 –82 kJmol–1
–82 kJmol–1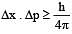
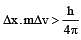
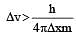

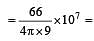 5.79 x106 m/sec
5.79 x106 m/sec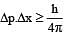
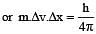 [∵ Δp= mΔv]
[∵ Δp= mΔv]