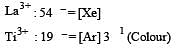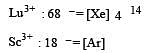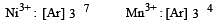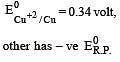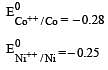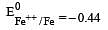31 Year NEET Previous Year Questions: The d & f-Block Elements - 1 - NEET MCQ
30 Questions MCQ Test Chemistry Class 12 - 31 Year NEET Previous Year Questions: The d & f-Block Elements - 1
Which of the following statement is not correct? [2001]
General electronic configuration of lanthanides is[2002]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following shows maximum number of oxidation states? [2002]
The basic character of the transition metal monoxides follows the order [2003] (Atomic Nos.,Ti = 22, V = 23, Cr = 24, Fe = 26)
Which one of the following characteristics of the transition metals is associated with their catalytic activity? [2003]
The correct order of ionic radii of Y3+, La3+, Eu3+ and Lu3+ is [2003]
Among the following series of transition metal ions, the one where all metal ions have 3d2 electronic configuration is(At. nos. Ti = 22; V = 23; Cr = 24; Mn = 25) [2004]
Lanthanoids are [2004]
The aqueous solution containing which one of the following ions will be colourless? (Atomic number: Sc = 21, Fe = 26, Ti = 22, Mn = 25)[2005]
The main reason for larger number of oxidation states exhibited by the actinoids than the corresponding lanthanoids, is [2005, 2006]
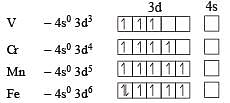
Four successive members of the first row transition elements are listed below with their atomic numbers. Which one of them is expected to have the highest third ionization enthalpy? [2 00 5]
Copper sulphate dissolves in excess of KCN to give[2006]
In which of the following pairs are both the ions coloured in aqueous solutions ? [2006] (At. no. : Sc = 21, Ti = 22, Ni = 28, Cu = 29, Co = 27)
Which of the following oxidation states are the most characteristic for lead and tin respectively?
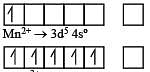
Which one of the following ions is the most stable in aqueous solution? [2007] (At.No. Ti = 22, V = 23, Cr = 24, Mn = 25)
Identify the incorrect statement among the following: [2007]
The correctorder of decreasing second ionisation enthalpy of Ti (22), V(23), Cr(24) and Mn (25) is : [2008]
Which one of the elements with the following outer orbital configurations may exhibit the largest number of oxidation states? [2009]
Qut of TiF62–, CoF63–, Cu2Cl2 and NiCl24– (Z of Ti = 22, Co = 27, Cu = 29, Ni = 28), the colourless species are: [2009]
Which of the following ions will exhibit colour in aqueous solutions? [2010]
Which one of the following ions has electronic configuration [Ar] 3d6 ? [2010]
(At. Nos. Mn = 25, Fe = 26, Co = 27, Ni = 28)
Which of the following pairs has the same size?
Which of the following oxidation states is the most common among the lanthanoids? [2010]
For the four successive transition elements (Cr, Mn, Fe and Co), the stability of +2 oxidation state will be there in which of the following order?
Acidified K2Cr2O7 solution turns green when Na2SO3 is added to it. This is due to the formation of : [2011]
Which of the statements is not true? [2012]
Which one of the following does not correctly represent the correct order of the property indicated against it? [2012 M]
Four successive members of the first series of the transition metals are listed below. For which one of them the standard potential (E0M2+ /M) value has a positive sign? [2012 M]
The catalytic activity of transition metals and their compounds is ascribed mainly to :[2012 M]
Which of the following exhibit only + 3 oxidation state ? [2012 M]
|
100 videos|282 docs|123 tests
|


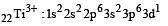
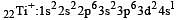
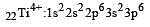
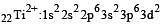
 Thus option (b) is discarded
Thus option (b) is discarded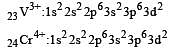
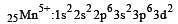
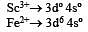

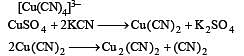


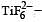 Ti is in + 4 O.S. ; 3d0 = colourless
Ti is in + 4 O.S. ; 3d0 = colourless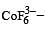 Co is in + 3 O.S ; 3d5 = coloured
Co is in + 3 O.S ; 3d5 = coloured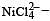 Ni is in + 2 O.S ; 3d8 – coloured
Ni is in + 2 O.S ; 3d8 – coloured