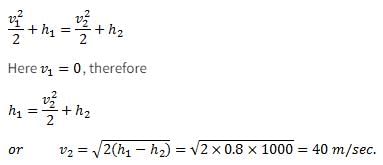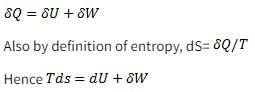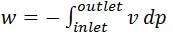Test: Basic Concept - 1 - Mechanical Engineering MCQ
15 Questions MCQ Test - Test: Basic Concept - 1
The sequence of processes that eventually returns the working substance to its original state is known as
According to thermodynamics, the universe is defined as_______.
Which one ofthe following is extensive property of a thermodynamics system
Which of the following quantities is not the property of the system
The value of an extensive property is extensively dependent on
Which of the following are intensive properties
1. Kinetic energy
2. Specific enthalpy
3. Pressure
4. Entropy
Codes:
For a system to be in thermodynamic equilibrium the system and its surroundings are to be in
A small steam whistle (perfectly insulated and doing no shaft work) causes a drop of 0.8 kJ/kg in enthalpy of steam from entry to exit. The kinetic energy of the steam at entry is negligible, the velocity of steam at exit is
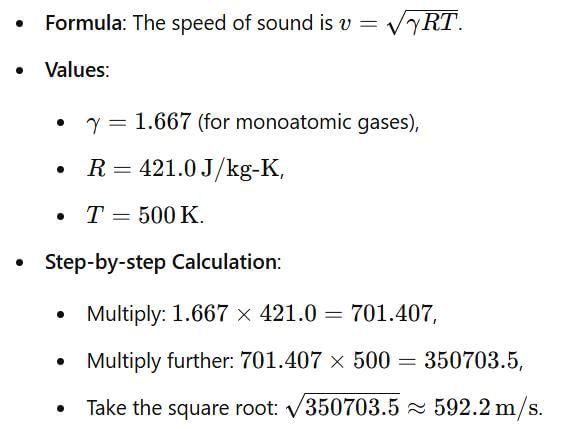
What is the speed of sound in Neon gas at a temperature of 500 K (gas constant of neon is 0.4210 kJ/kg-k)?
Which of the following relationship is valid only for reversible process undergone by a closed system of simple compressible substance (neglect changes in kinetic and potential energy)?
In a steady state flow process taking place in a device with a single inlet and a single outlet, the work done per unit mass flow rate is given by 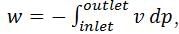 where v is the specific volume and p is the pressure. The expression for w given above
where v is the specific volume and p is the pressure. The expression for w given above
A balloon containing an ideal gas is initially kept in an evacuated and insulated room. The balloon ruptures and the gas fills up the entire room. Which one of the following statement is true at the end of the above process?