NEET Exam > NEET Questions > The half-life of a radioactive isotope &lsquo...
Start Learning for Free
The half-life of a radioactive isotope ‘X’ is 20 years. It decays to another element ‘Y’ which is stable. The
two elements ‘X’ and ‘Y’ were found to be in the ratio 1 : 7 in a sample of a given rock. The age of the rock
is estimated to be -
two elements ‘X’ and ‘Y’ were found to be in the ratio 1 : 7 in a sample of a given rock. The age of the rock
is estimated to be -
- a)40 years
- b)60 years
- c)80 years
- d)100 years
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
The half-life of a radioactive isotope ‘X’ is 20 years. It...
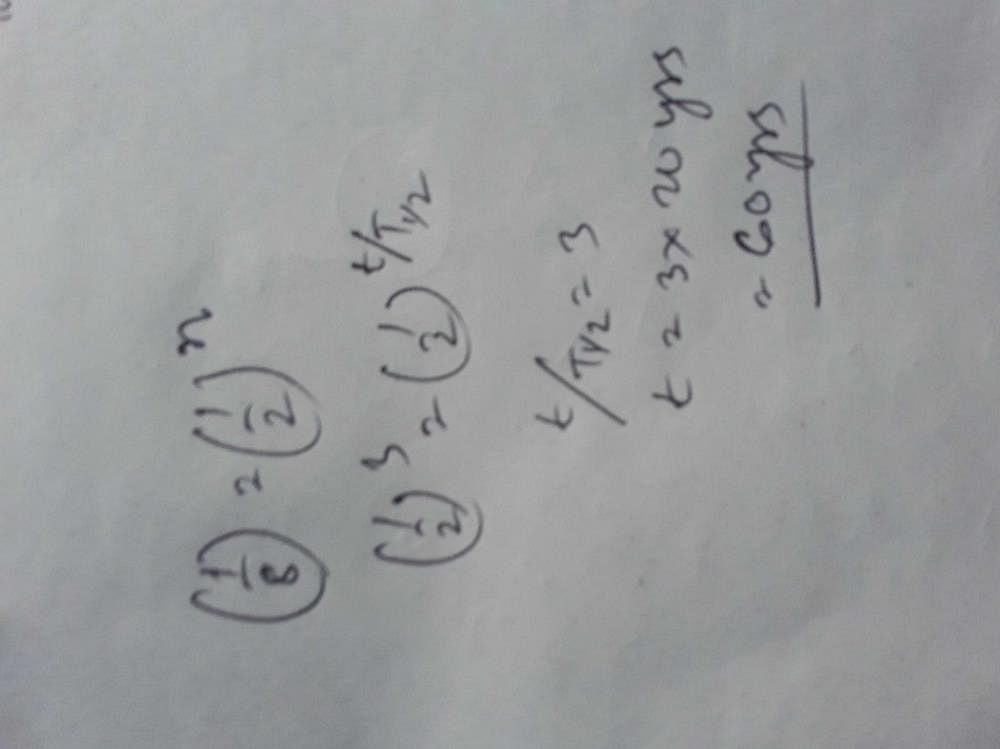
Free Test
FREE
| Start Free Test |
Community Answer
The half-life of a radioactive isotope ‘X’ is 20 years. It...
Is the time it takes for half of the atoms in a sample to decay. This decay process is random and cannot be influenced by external factors such as temperature or pressure. The half-life is a constant characteristic of each radioactive isotope and is unique to that isotope.
The concept of half-life is used to measure the stability or rate of decay of a radioactive material. It is commonly used in radiometric dating to determine the age of rocks, fossils, and archaeological artifacts. By measuring the ratio of the remaining radioactive isotope to the decay product, scientists can calculate the number of half-lives that have passed and determine the age of the sample.
For example, if the half-life of a radioactive isotope is 100 years, after 100 years, half of the atoms in the sample will have decayed, leaving half of the original amount. After another 100 years, half of the remaining atoms will decay, leaving only one-fourth of the original amount, and so on.
The half-life can vary widely depending on the isotope. Some isotopes have half-lives measured in seconds, while others have half-lives measured in billions of years. The most well-known example is carbon-14, which has a half-life of about 5,730 years and is commonly used in dating organic materials up to around 50,000 years old.
Understanding the half-life of a radioactive isotope is crucial in various scientific fields, including nuclear physics, geology, and archaeology. It allows scientists to determine the age of materials, study the behavior of radioactive substances, and make predictions about their potential risks and benefits.
The concept of half-life is used to measure the stability or rate of decay of a radioactive material. It is commonly used in radiometric dating to determine the age of rocks, fossils, and archaeological artifacts. By measuring the ratio of the remaining radioactive isotope to the decay product, scientists can calculate the number of half-lives that have passed and determine the age of the sample.
For example, if the half-life of a radioactive isotope is 100 years, after 100 years, half of the atoms in the sample will have decayed, leaving half of the original amount. After another 100 years, half of the remaining atoms will decay, leaving only one-fourth of the original amount, and so on.
The half-life can vary widely depending on the isotope. Some isotopes have half-lives measured in seconds, while others have half-lives measured in billions of years. The most well-known example is carbon-14, which has a half-life of about 5,730 years and is commonly used in dating organic materials up to around 50,000 years old.
Understanding the half-life of a radioactive isotope is crucial in various scientific fields, including nuclear physics, geology, and archaeology. It allows scientists to determine the age of materials, study the behavior of radioactive substances, and make predictions about their potential risks and benefits.
Attention NEET Students!
To make sure you are not studying endlessly, EduRev has designed NEET study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in NEET.

|
Explore Courses for NEET exam
|

|
Similar NEET Doubts
The half-life of a radioactive isotope ‘X’ is 20 years. It decays to another element ‘Y’ which is stable. Thetwo elements ‘X’ and ‘Y’ were found to be in the ratio 1 : 7 in a sample of a given rock. The age of the rockis estimated to be -a)40 yearsb)60 yearsc)80 yearsd)100 yearsCorrect answer is option 'B'. Can you explain this answer?
Question Description
The half-life of a radioactive isotope ‘X’ is 20 years. It decays to another element ‘Y’ which is stable. Thetwo elements ‘X’ and ‘Y’ were found to be in the ratio 1 : 7 in a sample of a given rock. The age of the rockis estimated to be -a)40 yearsb)60 yearsc)80 yearsd)100 yearsCorrect answer is option 'B'. Can you explain this answer? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about The half-life of a radioactive isotope ‘X’ is 20 years. It decays to another element ‘Y’ which is stable. Thetwo elements ‘X’ and ‘Y’ were found to be in the ratio 1 : 7 in a sample of a given rock. The age of the rockis estimated to be -a)40 yearsb)60 yearsc)80 yearsd)100 yearsCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The half-life of a radioactive isotope ‘X’ is 20 years. It decays to another element ‘Y’ which is stable. Thetwo elements ‘X’ and ‘Y’ were found to be in the ratio 1 : 7 in a sample of a given rock. The age of the rockis estimated to be -a)40 yearsb)60 yearsc)80 yearsd)100 yearsCorrect answer is option 'B'. Can you explain this answer?.
The half-life of a radioactive isotope ‘X’ is 20 years. It decays to another element ‘Y’ which is stable. Thetwo elements ‘X’ and ‘Y’ were found to be in the ratio 1 : 7 in a sample of a given rock. The age of the rockis estimated to be -a)40 yearsb)60 yearsc)80 yearsd)100 yearsCorrect answer is option 'B'. Can you explain this answer? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about The half-life of a radioactive isotope ‘X’ is 20 years. It decays to another element ‘Y’ which is stable. Thetwo elements ‘X’ and ‘Y’ were found to be in the ratio 1 : 7 in a sample of a given rock. The age of the rockis estimated to be -a)40 yearsb)60 yearsc)80 yearsd)100 yearsCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The half-life of a radioactive isotope ‘X’ is 20 years. It decays to another element ‘Y’ which is stable. Thetwo elements ‘X’ and ‘Y’ were found to be in the ratio 1 : 7 in a sample of a given rock. The age of the rockis estimated to be -a)40 yearsb)60 yearsc)80 yearsd)100 yearsCorrect answer is option 'B'. Can you explain this answer?.
Solutions for The half-life of a radioactive isotope ‘X’ is 20 years. It decays to another element ‘Y’ which is stable. Thetwo elements ‘X’ and ‘Y’ were found to be in the ratio 1 : 7 in a sample of a given rock. The age of the rockis estimated to be -a)40 yearsb)60 yearsc)80 yearsd)100 yearsCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of The half-life of a radioactive isotope ‘X’ is 20 years. It decays to another element ‘Y’ which is stable. Thetwo elements ‘X’ and ‘Y’ were found to be in the ratio 1 : 7 in a sample of a given rock. The age of the rockis estimated to be -a)40 yearsb)60 yearsc)80 yearsd)100 yearsCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The half-life of a radioactive isotope ‘X’ is 20 years. It decays to another element ‘Y’ which is stable. Thetwo elements ‘X’ and ‘Y’ were found to be in the ratio 1 : 7 in a sample of a given rock. The age of the rockis estimated to be -a)40 yearsb)60 yearsc)80 yearsd)100 yearsCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for The half-life of a radioactive isotope ‘X’ is 20 years. It decays to another element ‘Y’ which is stable. Thetwo elements ‘X’ and ‘Y’ were found to be in the ratio 1 : 7 in a sample of a given rock. The age of the rockis estimated to be -a)40 yearsb)60 yearsc)80 yearsd)100 yearsCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of The half-life of a radioactive isotope ‘X’ is 20 years. It decays to another element ‘Y’ which is stable. Thetwo elements ‘X’ and ‘Y’ were found to be in the ratio 1 : 7 in a sample of a given rock. The age of the rockis estimated to be -a)40 yearsb)60 yearsc)80 yearsd)100 yearsCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The half-life of a radioactive isotope ‘X’ is 20 years. It decays to another element ‘Y’ which is stable. Thetwo elements ‘X’ and ‘Y’ were found to be in the ratio 1 : 7 in a sample of a given rock. The age of the rockis estimated to be -a)40 yearsb)60 yearsc)80 yearsd)100 yearsCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























