CTET & State TET Exam > CTET & State TET Questions > Which of the following processes is required ...
Start Learning for Free
Which of the following processes is required for extracting metal from cinnabar ore?
- a)Roasting
- b)Electrolytic reduction
- c)Thermit process
- d)Calcination
Correct answer is option 'A'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
Which of the following processes is required for extracting metal from...
To extract metal from cinnabar ore, the process of roasting is required.
Roasting:
- Roasting is a process in which the ore is heated in the presence of excess air or oxygen.
- It is usually carried out in a furnace or kiln.
- The purpose of roasting is to convert the ore into an oxide form, which can then be easily reduced to obtain the metal.
- In the case of cinnabar ore, which is mercury sulfide (HgS), roasting is necessary to convert it into mercury oxide (HgO).
Steps in the Roasting Process:
1. Crushing and Grinding: The cinnabar ore is first crushed and ground to a fine powder to increase its surface area for better reaction with oxygen during roasting.
2. Heating: The powdered ore is then heated in a furnace or kiln. The temperature and duration of heating may vary depending on the specific ore and desired end product.
3. Oxidation: As the ore is heated, the sulfur in cinnabar combines with oxygen from the air to form sulfur dioxide (SO2) gas. This is known as the oxidation of sulfur.
- HgS + O2 → HgO + SO2
4. Reduction: The mercury oxide (HgO) formed in the previous step is then reduced to obtain metallic mercury (Hg).
- HgO → Hg + O2
- The reduction is usually carried out in a separate furnace or retort, where the mercury vaporizes and can be collected and condensed.
Importance of Roasting:
- Roasting is an important step in the extraction of metals from their ores as it helps in the conversion of the ore into a suitable form for further processing.
- In the case of cinnabar ore, roasting is necessary to convert mercury sulfide into mercury oxide, which can then be reduced to obtain metallic mercury.
- Without the roasting process, it would be difficult to extract mercury from cinnabar ore.
Other Processes:
- Electrolytic reduction, thermit process, and calcination are not suitable for extracting metal from cinnabar ore.
- Electrolytic reduction is used for extracting metals like aluminum and magnesium from their oxides.
- The thermit process is used for reducing metal oxides using aluminum as a reducing agent.
- Calcination is a process of heating the ore in the absence of air to remove volatile impurities or to convert it into a more stable oxide form.
Roasting:
- Roasting is a process in which the ore is heated in the presence of excess air or oxygen.
- It is usually carried out in a furnace or kiln.
- The purpose of roasting is to convert the ore into an oxide form, which can then be easily reduced to obtain the metal.
- In the case of cinnabar ore, which is mercury sulfide (HgS), roasting is necessary to convert it into mercury oxide (HgO).
Steps in the Roasting Process:
1. Crushing and Grinding: The cinnabar ore is first crushed and ground to a fine powder to increase its surface area for better reaction with oxygen during roasting.
2. Heating: The powdered ore is then heated in a furnace or kiln. The temperature and duration of heating may vary depending on the specific ore and desired end product.
3. Oxidation: As the ore is heated, the sulfur in cinnabar combines with oxygen from the air to form sulfur dioxide (SO2) gas. This is known as the oxidation of sulfur.
- HgS + O2 → HgO + SO2
4. Reduction: The mercury oxide (HgO) formed in the previous step is then reduced to obtain metallic mercury (Hg).
- HgO → Hg + O2
- The reduction is usually carried out in a separate furnace or retort, where the mercury vaporizes and can be collected and condensed.
Importance of Roasting:
- Roasting is an important step in the extraction of metals from their ores as it helps in the conversion of the ore into a suitable form for further processing.
- In the case of cinnabar ore, roasting is necessary to convert mercury sulfide into mercury oxide, which can then be reduced to obtain metallic mercury.
- Without the roasting process, it would be difficult to extract mercury from cinnabar ore.
Other Processes:
- Electrolytic reduction, thermit process, and calcination are not suitable for extracting metal from cinnabar ore.
- Electrolytic reduction is used for extracting metals like aluminum and magnesium from their oxides.
- The thermit process is used for reducing metal oxides using aluminum as a reducing agent.
- Calcination is a process of heating the ore in the absence of air to remove volatile impurities or to convert it into a more stable oxide form.
Free Test
FREE
| Start Free Test |
Community Answer
Which of the following processes is required for extracting metal from...
- Mineral: A naturally occurring substance with uniform chemical composition is called a mineral.
- Ore: An ore is a mineral from which a metal can be extracted in a profitable way.
According to the reactivity of metals, different methods are employed to extract metals from their ores.
Reactivity series of metals:
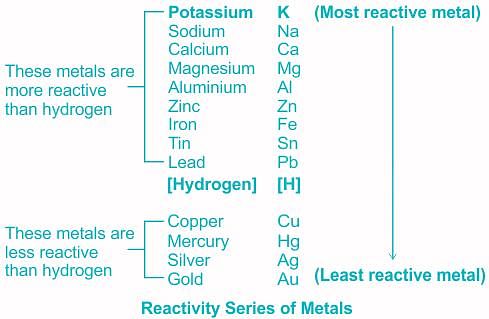
Reactivity series of metals:

- Metals of high reactivity; such as sodium, calcium, magnesium, aluminium, etc. are extracted from their ores by electrolytic reduction.
- Metals of middle reactivity, Iron, zinc, lead, etc. are found in the form of carbonate or sulfide ores. They are either calcinated or roasted.
- Metals of least reactivity, Mercury and copper, which belong to the least reactivity series, are often found in the form of their sulfide ores. They are roasted and then refined.
Attention CTET & State TET Students!
To make sure you are not studying endlessly, EduRev has designed CTET & State TET study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in CTET & State TET.

|
Explore Courses for CTET & State TET exam
|

|
Which of the following processes is required for extracting metal from cinnabar ore?a)Roastingb)Electrolytic reductionc)Thermit processd)CalcinationCorrect answer is option 'A'. Can you explain this answer?
Question Description
Which of the following processes is required for extracting metal from cinnabar ore?a)Roastingb)Electrolytic reductionc)Thermit processd)CalcinationCorrect answer is option 'A'. Can you explain this answer? for CTET & State TET 2024 is part of CTET & State TET preparation. The Question and answers have been prepared according to the CTET & State TET exam syllabus. Information about Which of the following processes is required for extracting metal from cinnabar ore?a)Roastingb)Electrolytic reductionc)Thermit processd)CalcinationCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for CTET & State TET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following processes is required for extracting metal from cinnabar ore?a)Roastingb)Electrolytic reductionc)Thermit processd)CalcinationCorrect answer is option 'A'. Can you explain this answer?.
Which of the following processes is required for extracting metal from cinnabar ore?a)Roastingb)Electrolytic reductionc)Thermit processd)CalcinationCorrect answer is option 'A'. Can you explain this answer? for CTET & State TET 2024 is part of CTET & State TET preparation. The Question and answers have been prepared according to the CTET & State TET exam syllabus. Information about Which of the following processes is required for extracting metal from cinnabar ore?a)Roastingb)Electrolytic reductionc)Thermit processd)CalcinationCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for CTET & State TET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following processes is required for extracting metal from cinnabar ore?a)Roastingb)Electrolytic reductionc)Thermit processd)CalcinationCorrect answer is option 'A'. Can you explain this answer?.
Solutions for Which of the following processes is required for extracting metal from cinnabar ore?a)Roastingb)Electrolytic reductionc)Thermit processd)CalcinationCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for CTET & State TET.
Download more important topics, notes, lectures and mock test series for CTET & State TET Exam by signing up for free.
Here you can find the meaning of Which of the following processes is required for extracting metal from cinnabar ore?a)Roastingb)Electrolytic reductionc)Thermit processd)CalcinationCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which of the following processes is required for extracting metal from cinnabar ore?a)Roastingb)Electrolytic reductionc)Thermit processd)CalcinationCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for Which of the following processes is required for extracting metal from cinnabar ore?a)Roastingb)Electrolytic reductionc)Thermit processd)CalcinationCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of Which of the following processes is required for extracting metal from cinnabar ore?a)Roastingb)Electrolytic reductionc)Thermit processd)CalcinationCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which of the following processes is required for extracting metal from cinnabar ore?a)Roastingb)Electrolytic reductionc)Thermit processd)CalcinationCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice CTET & State TET tests.

|
Explore Courses for CTET & State TET exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.