MCAT Exam > MCAT Questions > Cis-platin [PtCl2(NH3)2] is an anti-cancer dr...
Start Learning for Free
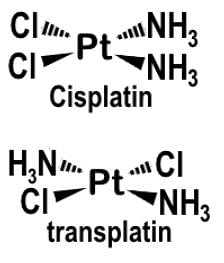
Cis-platin [PtCl2(NH3)2] is an anti-cancer drug that reacts by binding to and causing the crosslinking of DNA ultimately leading to apoptosis and is the geometric isomer of trans-platin. Given that cis-platin exhibits stereochemistry, what would you predict is the molecular geometry of the anticancer drug?
- a)trigonal pyramidal
- b)square pyramidal
- c)square planar
- d)tetrahedral
Correct answer is option 'C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
Cis-platin [PtCl2(NH3)2] is an anti-cancer drug that reacts by binding...
In cis-platin, platinum is in a Pt(II) form, which means that platinum still has a d8 configuration. Additionally, each of the ligands donates both electrons to form the coordinate covalent bond, so platinum has a 16 electron configuration. That is not necessary to answer the question since we are looking for a molecular geometry.
What determines the molecular geometry is the number of atoms attached, and here the ligands. Cis-platin has four ligands attached, 2 NH3 and 2 Cl‾.
We need a molecular geometry that allows for only 4 attached atoms or ligands, and tetrahedral and square planar fits that bill. Square pyramidal and trigonal pyramidal are geometries with 5 and 3 attached atoms, respectively.
In order for the molecule to have a stereocenter, only tetrahedral, square pyramidal, and square planar would work as the molecular geometry. Tetrahedral would give a stereocenter if all four substituents or attached atoms were different, and square pyramidal once again needs 5 attached atoms or ligands.
Additionally, there are 4 lone pairs as well as four ligands. With four lone pairs, square planar and square pyramidal are the only configurations that can accommodate four electron pairs.
Only in the square planar configuration will the following geometric isomers form:
Free Test
FREE
| Start Free Test |
Community Answer
Cis-platin [PtCl2(NH3)2] is an anti-cancer drug that reacts by binding...
Explanation:
In order to determine the molecular geometry of cis-platin [PtCl2(NH3)2], we need to consider the arrangement of atoms around the central metal atom (platinum, Pt) and the presence of any lone pairs of electrons.
1. Central Atom:
The central atom in cis-platin is platinum (Pt).
2. Ligands:
The ligands in cis-platin are two chloride ions (Cl-) and two ammonia molecules (NH3).
3. Coordination Number:
The coordination number of platinum is determined by the number of ligands surrounding it. In this case, there are four ligands (two chloride ions and two ammonia molecules), so the coordination number is 4.
4. Lone Pairs:
In cis-platin, the central platinum atom does not have any lone pairs of electrons.
5. Geometry:
Based on the coordination number and absence of lone pairs, the molecular geometry of cis-platin can be determined using the VSEPR theory (Valence Shell Electron Pair Repulsion theory).
Since there are four ligands (two chloride ions and two ammonia molecules) and no lone pairs, the electronic geometry of cis-platin is tetrahedral.
However, the question asks for the molecular geometry, which takes into account the presence of multiple ligands. In cis-platin, the two chloride ions and the two ammonia molecules are not symmetrical around the central platinum atom.
This lack of symmetry results in the cis arrangement, where the chloride ions and ammonia molecules are adjacent to each other rather than across from each other. This cis arrangement gives cis-platin its geometric isomerism.
The cis arrangement leads to the molecular geometry of square planar. In a square planar geometry, the ligands are arranged in a square plane around the central atom, with bond angles of 90 degrees.
Therefore, the correct answer is option C: square planar.
In order to determine the molecular geometry of cis-platin [PtCl2(NH3)2], we need to consider the arrangement of atoms around the central metal atom (platinum, Pt) and the presence of any lone pairs of electrons.
1. Central Atom:
The central atom in cis-platin is platinum (Pt).
2. Ligands:
The ligands in cis-platin are two chloride ions (Cl-) and two ammonia molecules (NH3).
3. Coordination Number:
The coordination number of platinum is determined by the number of ligands surrounding it. In this case, there are four ligands (two chloride ions and two ammonia molecules), so the coordination number is 4.
4. Lone Pairs:
In cis-platin, the central platinum atom does not have any lone pairs of electrons.
5. Geometry:
Based on the coordination number and absence of lone pairs, the molecular geometry of cis-platin can be determined using the VSEPR theory (Valence Shell Electron Pair Repulsion theory).
Since there are four ligands (two chloride ions and two ammonia molecules) and no lone pairs, the electronic geometry of cis-platin is tetrahedral.
However, the question asks for the molecular geometry, which takes into account the presence of multiple ligands. In cis-platin, the two chloride ions and the two ammonia molecules are not symmetrical around the central platinum atom.
This lack of symmetry results in the cis arrangement, where the chloride ions and ammonia molecules are adjacent to each other rather than across from each other. This cis arrangement gives cis-platin its geometric isomerism.
The cis arrangement leads to the molecular geometry of square planar. In a square planar geometry, the ligands are arranged in a square plane around the central atom, with bond angles of 90 degrees.
Therefore, the correct answer is option C: square planar.
Attention MCAT Students!
To make sure you are not studying endlessly, EduRev has designed MCAT study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in MCAT.

|
Explore Courses for MCAT exam
|

|
Similar MCAT Doubts
Cis-platin [PtCl2(NH3)2] is an anti-cancer drug that reacts by binding to and causing the crosslinking of DNA ultimately leading to apoptosis and is the geometric isomer of trans-platin. Given that cis-platin exhibits stereochemistry, what would you predict is the molecular geometry of the anticancer drug?a)trigonal pyramidalb)square pyramidalc)square planard)tetrahedralCorrect answer is option 'C'. Can you explain this answer?
Question Description
Cis-platin [PtCl2(NH3)2] is an anti-cancer drug that reacts by binding to and causing the crosslinking of DNA ultimately leading to apoptosis and is the geometric isomer of trans-platin. Given that cis-platin exhibits stereochemistry, what would you predict is the molecular geometry of the anticancer drug?a)trigonal pyramidalb)square pyramidalc)square planard)tetrahedralCorrect answer is option 'C'. Can you explain this answer? for MCAT 2024 is part of MCAT preparation. The Question and answers have been prepared according to the MCAT exam syllabus. Information about Cis-platin [PtCl2(NH3)2] is an anti-cancer drug that reacts by binding to and causing the crosslinking of DNA ultimately leading to apoptosis and is the geometric isomer of trans-platin. Given that cis-platin exhibits stereochemistry, what would you predict is the molecular geometry of the anticancer drug?a)trigonal pyramidalb)square pyramidalc)square planard)tetrahedralCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for MCAT 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Cis-platin [PtCl2(NH3)2] is an anti-cancer drug that reacts by binding to and causing the crosslinking of DNA ultimately leading to apoptosis and is the geometric isomer of trans-platin. Given that cis-platin exhibits stereochemistry, what would you predict is the molecular geometry of the anticancer drug?a)trigonal pyramidalb)square pyramidalc)square planard)tetrahedralCorrect answer is option 'C'. Can you explain this answer?.
Cis-platin [PtCl2(NH3)2] is an anti-cancer drug that reacts by binding to and causing the crosslinking of DNA ultimately leading to apoptosis and is the geometric isomer of trans-platin. Given that cis-platin exhibits stereochemistry, what would you predict is the molecular geometry of the anticancer drug?a)trigonal pyramidalb)square pyramidalc)square planard)tetrahedralCorrect answer is option 'C'. Can you explain this answer? for MCAT 2024 is part of MCAT preparation. The Question and answers have been prepared according to the MCAT exam syllabus. Information about Cis-platin [PtCl2(NH3)2] is an anti-cancer drug that reacts by binding to and causing the crosslinking of DNA ultimately leading to apoptosis and is the geometric isomer of trans-platin. Given that cis-platin exhibits stereochemistry, what would you predict is the molecular geometry of the anticancer drug?a)trigonal pyramidalb)square pyramidalc)square planard)tetrahedralCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for MCAT 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Cis-platin [PtCl2(NH3)2] is an anti-cancer drug that reacts by binding to and causing the crosslinking of DNA ultimately leading to apoptosis and is the geometric isomer of trans-platin. Given that cis-platin exhibits stereochemistry, what would you predict is the molecular geometry of the anticancer drug?a)trigonal pyramidalb)square pyramidalc)square planard)tetrahedralCorrect answer is option 'C'. Can you explain this answer?.
Solutions for Cis-platin [PtCl2(NH3)2] is an anti-cancer drug that reacts by binding to and causing the crosslinking of DNA ultimately leading to apoptosis and is the geometric isomer of trans-platin. Given that cis-platin exhibits stereochemistry, what would you predict is the molecular geometry of the anticancer drug?a)trigonal pyramidalb)square pyramidalc)square planard)tetrahedralCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for MCAT.
Download more important topics, notes, lectures and mock test series for MCAT Exam by signing up for free.
Here you can find the meaning of Cis-platin [PtCl2(NH3)2] is an anti-cancer drug that reacts by binding to and causing the crosslinking of DNA ultimately leading to apoptosis and is the geometric isomer of trans-platin. Given that cis-platin exhibits stereochemistry, what would you predict is the molecular geometry of the anticancer drug?a)trigonal pyramidalb)square pyramidalc)square planard)tetrahedralCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Cis-platin [PtCl2(NH3)2] is an anti-cancer drug that reacts by binding to and causing the crosslinking of DNA ultimately leading to apoptosis and is the geometric isomer of trans-platin. Given that cis-platin exhibits stereochemistry, what would you predict is the molecular geometry of the anticancer drug?a)trigonal pyramidalb)square pyramidalc)square planard)tetrahedralCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for Cis-platin [PtCl2(NH3)2] is an anti-cancer drug that reacts by binding to and causing the crosslinking of DNA ultimately leading to apoptosis and is the geometric isomer of trans-platin. Given that cis-platin exhibits stereochemistry, what would you predict is the molecular geometry of the anticancer drug?a)trigonal pyramidalb)square pyramidalc)square planard)tetrahedralCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of Cis-platin [PtCl2(NH3)2] is an anti-cancer drug that reacts by binding to and causing the crosslinking of DNA ultimately leading to apoptosis and is the geometric isomer of trans-platin. Given that cis-platin exhibits stereochemistry, what would you predict is the molecular geometry of the anticancer drug?a)trigonal pyramidalb)square pyramidalc)square planard)tetrahedralCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Cis-platin [PtCl2(NH3)2] is an anti-cancer drug that reacts by binding to and causing the crosslinking of DNA ultimately leading to apoptosis and is the geometric isomer of trans-platin. Given that cis-platin exhibits stereochemistry, what would you predict is the molecular geometry of the anticancer drug?a)trigonal pyramidalb)square pyramidalc)square planard)tetrahedralCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice MCAT tests.

|
Explore Courses for MCAT exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.