Class 9 Exam > Class 9 Questions > What is the difference between amorphous and ...
Start Learning for Free
What is the difference between amorphous and crystalline solids?
Verified Answer
What is the difference between amorphous and crystalline solids?
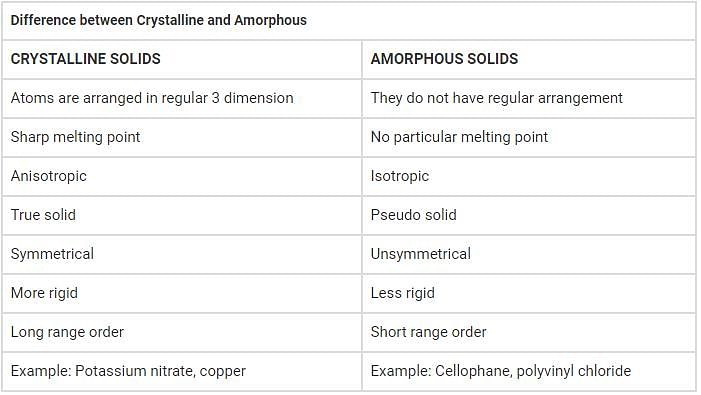
 This question is part of UPSC exam. View all Class 9 courses
This question is part of UPSC exam. View all Class 9 courses
Most Upvoted Answer
What is the difference between amorphous and crystalline solids?
Amorphous and Crystalline Solids: Understanding the Difference
Introduction:
Solids are one of the three states of matter, along with liquids and gases. They are characterized by their definite shape and volume. Solids can be broadly classified into two categories: amorphous solids and crystalline solids. While both these types of solids are made up of atoms or molecules, they differ in their arrangement and properties.
Amorphous Solids:
Amorphous solids are characterized by a lack of long-range order in their atomic or molecular arrangement. This means that the constituent particles are randomly and irregularly arranged, resulting in a disordered structure. Some examples of amorphous solids include glass, rubber, and plastic.
Properties of Amorphous Solids:
1. Lack of definite melting point: Amorphous solids do not have a distinct melting point. Instead, they gradually soften and become more fluid as the temperature increases.
2. Isotropic nature: Amorphous solids have an isotropic nature, meaning their properties are the same in all directions. This is because there is no preferred direction or orientation of the particles.
3. Lack of cleavage: Due to the absence of a regular arrangement, amorphous solids do not exhibit cleavage. They fracture irregularly when subjected to external forces.
4. Variable mechanical properties: The mechanical properties of amorphous solids can vary widely depending on factors such as temperature and composition. This makes them more flexible and less brittle compared to crystalline solids.
Crystalline Solids:
Crystalline solids are characterized by a highly ordered and repetitive arrangement of atoms or molecules. This regular arrangement extends over long distances, giving rise to well-defined crystal structures. Examples of crystalline solids include salt, sugar, and diamond.
Properties of Crystalline Solids:
1. Definite melting point: Crystalline solids have a specific melting point, where they change from a solid to a liquid state. This melting point remains constant as long as the pressure is constant.
2. Anisotropic nature: Crystalline solids are anisotropic, meaning their properties vary with direction. This is because the arrangement of particles has a preferred direction, resulting in different properties along different crystallographic axes.
3. Cleavage: Crystalline solids often exhibit cleavage, which refers to the tendency to break along specific planes of weakness. This is due to the regular arrangement of particles in the crystal lattice.
4. Fixed mechanical properties: Crystalline solids have fixed mechanical properties that are determined by their crystal structure. These properties include hardness, brittleness, and elasticity.
Conclusion:
In summary, amorphous solids lack long-range order and have random arrangements of particles, while crystalline solids have a highly ordered and repetitive arrangement. The properties of these solids, such as melting point, cleavage, and mechanical behavior, differ based on their structure. Understanding the differences between amorphous and crystalline solids is important as it affects their physical, chemical, and mechanical properties, making them suitable for various applications in different fields.
Introduction:
Solids are one of the three states of matter, along with liquids and gases. They are characterized by their definite shape and volume. Solids can be broadly classified into two categories: amorphous solids and crystalline solids. While both these types of solids are made up of atoms or molecules, they differ in their arrangement and properties.
Amorphous Solids:
Amorphous solids are characterized by a lack of long-range order in their atomic or molecular arrangement. This means that the constituent particles are randomly and irregularly arranged, resulting in a disordered structure. Some examples of amorphous solids include glass, rubber, and plastic.
Properties of Amorphous Solids:
1. Lack of definite melting point: Amorphous solids do not have a distinct melting point. Instead, they gradually soften and become more fluid as the temperature increases.
2. Isotropic nature: Amorphous solids have an isotropic nature, meaning their properties are the same in all directions. This is because there is no preferred direction or orientation of the particles.
3. Lack of cleavage: Due to the absence of a regular arrangement, amorphous solids do not exhibit cleavage. They fracture irregularly when subjected to external forces.
4. Variable mechanical properties: The mechanical properties of amorphous solids can vary widely depending on factors such as temperature and composition. This makes them more flexible and less brittle compared to crystalline solids.
Crystalline Solids:
Crystalline solids are characterized by a highly ordered and repetitive arrangement of atoms or molecules. This regular arrangement extends over long distances, giving rise to well-defined crystal structures. Examples of crystalline solids include salt, sugar, and diamond.
Properties of Crystalline Solids:
1. Definite melting point: Crystalline solids have a specific melting point, where they change from a solid to a liquid state. This melting point remains constant as long as the pressure is constant.
2. Anisotropic nature: Crystalline solids are anisotropic, meaning their properties vary with direction. This is because the arrangement of particles has a preferred direction, resulting in different properties along different crystallographic axes.
3. Cleavage: Crystalline solids often exhibit cleavage, which refers to the tendency to break along specific planes of weakness. This is due to the regular arrangement of particles in the crystal lattice.
4. Fixed mechanical properties: Crystalline solids have fixed mechanical properties that are determined by their crystal structure. These properties include hardness, brittleness, and elasticity.
Conclusion:
In summary, amorphous solids lack long-range order and have random arrangements of particles, while crystalline solids have a highly ordered and repetitive arrangement. The properties of these solids, such as melting point, cleavage, and mechanical behavior, differ based on their structure. Understanding the differences between amorphous and crystalline solids is important as it affects their physical, chemical, and mechanical properties, making them suitable for various applications in different fields.
Community Answer
What is the difference between amorphous and crystalline solids?
Amorphous solids are not systematic in order.
Crystaline solids are systematic in order.
Crystaline solids are systematic in order.
Attention Class 9 Students!
To make sure you are not studying endlessly, EduRev has designed Class 9 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 9.

|
Explore Courses for Class 9 exam
|

|
Similar Class 9 Doubts
What is the difference between amorphous and crystalline solids?
Question Description
What is the difference between amorphous and crystalline solids? for Class 9 2024 is part of Class 9 preparation. The Question and answers have been prepared according to the Class 9 exam syllabus. Information about What is the difference between amorphous and crystalline solids? covers all topics & solutions for Class 9 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What is the difference between amorphous and crystalline solids?.
What is the difference between amorphous and crystalline solids? for Class 9 2024 is part of Class 9 preparation. The Question and answers have been prepared according to the Class 9 exam syllabus. Information about What is the difference between amorphous and crystalline solids? covers all topics & solutions for Class 9 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What is the difference between amorphous and crystalline solids?.
Solutions for What is the difference between amorphous and crystalline solids? in English & in Hindi are available as part of our courses for Class 9.
Download more important topics, notes, lectures and mock test series for Class 9 Exam by signing up for free.
Here you can find the meaning of What is the difference between amorphous and crystalline solids? defined & explained in the simplest way possible. Besides giving the explanation of
What is the difference between amorphous and crystalline solids?, a detailed solution for What is the difference between amorphous and crystalline solids? has been provided alongside types of What is the difference between amorphous and crystalline solids? theory, EduRev gives you an
ample number of questions to practice What is the difference between amorphous and crystalline solids? tests, examples and also practice Class 9 tests.

|
Explore Courses for Class 9 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























