IIT JAM Exam > IIT JAM Questions > why 3°amines is optically inactive? Related: ...
Start Learning for Free
why 3°amines is optically inactive?
Verified Answer
why 3°amines is optically inactive? Related: Stereochemistry - Stereo...
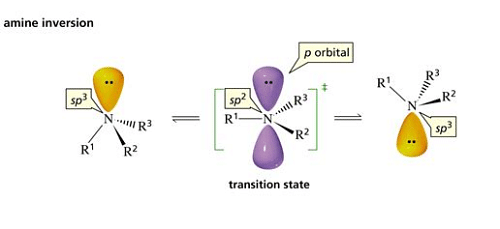
The inversion occurs because the nitrogen atom can rehybridize to a planar
sp2
geometry and then rehybridize to a tetrahedral
sp3
geometry with the opposite configuration.
The result is an optically inactive racemic mixture of the two rapidly-interconverting enantiomeric forms.
The activation energy for inversion is low, so the inversion rate at room temperature for many 3digree amines ranges from.
 This question is part of UPSC exam. View all IIT JAM courses
This question is part of UPSC exam. View all IIT JAM courses
Most Upvoted Answer
why 3°amines is optically inactive? Related: Stereochemistry - Stereo...
Introduction:
Optical activity refers to the ability of a compound to rotate the plane of polarized light. Chiral compounds, which have a non-superimposable mirror image, exhibit optical activity. However, certain compounds, such as tertiary amines, are optically inactive despite having chiral centers. This can be explained by considering the stereochemistry of tertiary amines.
Stereochemistry of Tertiary Amines:
Tertiary amines are compounds that contain a nitrogen atom bonded to three alkyl or aryl groups. Due to the presence of three bulky substituents around the nitrogen atom, the spatial arrangement of these groups prevents the formation of a chiral center. This is because a chiral center requires four different substituents bonded to a central atom.
Chirality and Optical Activity:
Chirality is a property of compounds that lack internal symmetry and have a mirror image that cannot be superimposed onto the original molecule. Chiral compounds exist as enantiomers, which are non-superimposable mirror images of each other. Enantiomers rotate plane-polarized light in equal amounts but in opposite directions, resulting in optical activity.
Reasons for Optical Inactivity of Tertiary Amines:
1. Lack of Chiral Centers: Tertiary amines lack a chiral center since the nitrogen atom is bonded to three identical alkyl or aryl groups. This symmetry prevents the formation of non-superimposable mirror images and thus optical activity.
2. Internal Compensation: In chiral compounds, the presence of an asymmetric carbon atom leads to an imbalance of substituents, resulting in optical activity. However, tertiary amines do not have this imbalance due to identical substituents around the nitrogen atom. Consequently, any rotation of plane-polarized light caused by one substituent is canceled out by the other two substituents, resulting in optical inactivity.
3. Plane of Symmetry: Tertiary amines often possess a plane of symmetry, which divides the molecule into two identical halves. This symmetry element ensures that any rotation of plane-polarized light caused by one half of the molecule is canceled out by the other half, making the compound optically inactive.
Conclusion:
Tertiary amines are optically inactive due to the absence of a chiral center and the presence of internal compensation and symmetry elements. These factors prevent the compound from exhibiting optical activity, despite having three different substituents attached to the nitrogen atom. Understanding the stereochemistry of tertiary amines helps in explaining their optical inactivity.
Optical activity refers to the ability of a compound to rotate the plane of polarized light. Chiral compounds, which have a non-superimposable mirror image, exhibit optical activity. However, certain compounds, such as tertiary amines, are optically inactive despite having chiral centers. This can be explained by considering the stereochemistry of tertiary amines.
Stereochemistry of Tertiary Amines:
Tertiary amines are compounds that contain a nitrogen atom bonded to three alkyl or aryl groups. Due to the presence of three bulky substituents around the nitrogen atom, the spatial arrangement of these groups prevents the formation of a chiral center. This is because a chiral center requires four different substituents bonded to a central atom.
Chirality and Optical Activity:
Chirality is a property of compounds that lack internal symmetry and have a mirror image that cannot be superimposed onto the original molecule. Chiral compounds exist as enantiomers, which are non-superimposable mirror images of each other. Enantiomers rotate plane-polarized light in equal amounts but in opposite directions, resulting in optical activity.
Reasons for Optical Inactivity of Tertiary Amines:
1. Lack of Chiral Centers: Tertiary amines lack a chiral center since the nitrogen atom is bonded to three identical alkyl or aryl groups. This symmetry prevents the formation of non-superimposable mirror images and thus optical activity.
2. Internal Compensation: In chiral compounds, the presence of an asymmetric carbon atom leads to an imbalance of substituents, resulting in optical activity. However, tertiary amines do not have this imbalance due to identical substituents around the nitrogen atom. Consequently, any rotation of plane-polarized light caused by one substituent is canceled out by the other two substituents, resulting in optical inactivity.
3. Plane of Symmetry: Tertiary amines often possess a plane of symmetry, which divides the molecule into two identical halves. This symmetry element ensures that any rotation of plane-polarized light caused by one half of the molecule is canceled out by the other half, making the compound optically inactive.
Conclusion:
Tertiary amines are optically inactive due to the absence of a chiral center and the presence of internal compensation and symmetry elements. These factors prevent the compound from exhibiting optical activity, despite having three different substituents attached to the nitrogen atom. Understanding the stereochemistry of tertiary amines helps in explaining their optical inactivity.

|
Explore Courses for IIT JAM exam
|

|
Similar IIT JAM Doubts
why 3°amines is optically inactive? Related: Stereochemistry - Stereochemistry, Organic Chemistry, IIT JAM
Question Description
why 3°amines is optically inactive? Related: Stereochemistry - Stereochemistry, Organic Chemistry, IIT JAM for IIT JAM 2024 is part of IIT JAM preparation. The Question and answers have been prepared according to the IIT JAM exam syllabus. Information about why 3°amines is optically inactive? Related: Stereochemistry - Stereochemistry, Organic Chemistry, IIT JAM covers all topics & solutions for IIT JAM 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for why 3°amines is optically inactive? Related: Stereochemistry - Stereochemistry, Organic Chemistry, IIT JAM.
why 3°amines is optically inactive? Related: Stereochemistry - Stereochemistry, Organic Chemistry, IIT JAM for IIT JAM 2024 is part of IIT JAM preparation. The Question and answers have been prepared according to the IIT JAM exam syllabus. Information about why 3°amines is optically inactive? Related: Stereochemistry - Stereochemistry, Organic Chemistry, IIT JAM covers all topics & solutions for IIT JAM 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for why 3°amines is optically inactive? Related: Stereochemistry - Stereochemistry, Organic Chemistry, IIT JAM.
Solutions for why 3°amines is optically inactive? Related: Stereochemistry - Stereochemistry, Organic Chemistry, IIT JAM in English & in Hindi are available as part of our courses for IIT JAM.
Download more important topics, notes, lectures and mock test series for IIT JAM Exam by signing up for free.
Here you can find the meaning of why 3°amines is optically inactive? Related: Stereochemistry - Stereochemistry, Organic Chemistry, IIT JAM defined & explained in the simplest way possible. Besides giving the explanation of
why 3°amines is optically inactive? Related: Stereochemistry - Stereochemistry, Organic Chemistry, IIT JAM, a detailed solution for why 3°amines is optically inactive? Related: Stereochemistry - Stereochemistry, Organic Chemistry, IIT JAM has been provided alongside types of why 3°amines is optically inactive? Related: Stereochemistry - Stereochemistry, Organic Chemistry, IIT JAM theory, EduRev gives you an
ample number of questions to practice why 3°amines is optically inactive? Related: Stereochemistry - Stereochemistry, Organic Chemistry, IIT JAM tests, examples and also practice IIT JAM tests.

|
Explore Courses for IIT JAM exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.



















