(VSEPR) Theory & Shapes of Molecules | Inorganic Chemistry PDF Download
VALENCE SHELL ELECTRON PAIR REPULSION (VSEPR) THEORY
According to the VSEPR model, molecules adopt geometries in which their valence electron pairs position themselves as far from each other as possible. A molecule can be described by the general formula AXmEn, where A is the central atom, X stands for any atom or group of atoms surrounding the central atom, and E represents lone pair of electron.
The steric number (SN = m + n) is the number of positions occupied by atoms or lone pairs around a central atom; lone pairs and bonds are nearly equal in their influence on molecular shape.
VSEPR Rules:
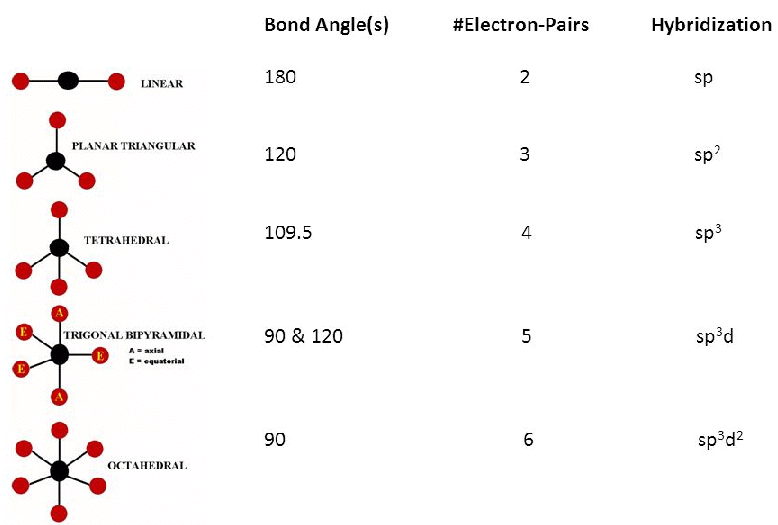
1. Electron pairs tend to minimize repulsions. Ideal geometries are:
(a) for two electron pairs, linear.
(b) for three electron pairs, trigonal.
(c) for four electron pairs, tetrahedral.
(d) for five electron pairs, trigonal bipyramidal.
(e) for sixelectron pairs, octahedral.2. Repulsions are of the order LP–LP > LP – BP > BP – BP.
(a) When lone pairs are present, the bond angles are smaller than predicted.
(b) Lone pairs choose the largest site, e.g., equatorial in trigonal bipyramid.
(c) If all sites are equal, lone pairs will be trans to each other.3. Double bond occupy more space than single bonds.
4. Bonding pairs to electronegative substituents occupy less space than those to more electropositive substituents. To the above Drago suggested the following empirical rule which rationalizes the very small angles (~90º) in phosphine, arsine, hydrogen sulfide, etc. and which is compatible with the energetics of hydridization.
5. If the central atom is in the third row or below in the periodic table, the lone pair will occupy a stereochemically inactive s-orbital and the bonding will be through p-orbitals and near 90º bond angles if the substituent electronegativity is ≤ ~ 2.5.
Bent Rules
If the electronegativity difference between the bonding moieties (i.e. central atom and other bonding atom or group where the central atom is less electronegative) is significantly large, then the corresponding bond will possess some ionic character. This will lead to the shifting of bonding electron pair to the more electronegative terminal atom or group.
This electron shifting is favoured more if the central atom utilises a hybrid orbital having less s-character. Thus, with the increase of difference of electronegativity between the combining species, the covalency decreases.
In such cases, the central atom uses the hybrid orbitals having less s-character towards the more electronegative bonding moiety, because utilisation of such hybrid orbitals favours the generation of more ionic character.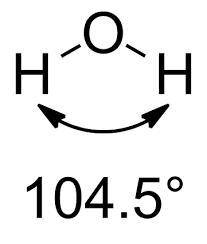 Bent rule
Bent rule
Illustration of the Bent’s Rule :
(i) H3CF: In a perfectly tetrahedral system (i.e. sp3 hybridisation) in each bond, C projects 75% pcharacter and 25% s-character. The bond angle is 109º28’. Increase of s-character and decrease of p-character widen the bond angle (cf. 180º for sp, 120º for sp2, 109º28’ for sp3).
Here, the CF bond should have less than 25% s-character of C and consequently the C-H bonds should have more than 25% s-character of C. Thus, the less p-character (i.e. < 75%) in the C-H bonds tends to extend the H-C-H angle greater than 109º28’.
Besides, the greater s-character in the C-H bonds tends to shorten the C-H bonds. This enhances the bond pair-bond pair repulsion to widen the H-C-H bond angle.
(ii) PCl3F2: The molecule has got a trigonal bipyramid (TBP) structure. The two fluorine atoms preferebly are positioned in the axial direction. The axially oriented hybrid orbitals (i.e. pd; Pz + d z2 ) having no s-character bind the more electronegative substituent, i.e. F.
The tendency of more electronegative substituents to occupy the axial or apical pd-hybrid orbitals of low electronegativity in TBP geometries is referred to as apicophilicity.
In MePF4, Me2PF3 the more electronegativity F-atoms tend to occupy the axial positions while the Me-groups of low electronegativity occupy the equatorial positions.
(iii) CH3 radical vs. CF3 radical:
The CH3 radical is planar and carbon is sp2 hybridised to house the odd unpaired electron in a pure p-orbital. On the other hand, CF3 radical is pyramidal i.e. here carbon is sp3 hybridised and the unpaired electron is housed in a sp3-hybrid orbital.
This difference can be explained by considering the Bent’s rule. Fluorine is more electronegative than hydrogen. This is why, fluorine directs carbon to project the s-p-hybrid orbital having more p-character.
Between sp3 (75% p-character) and sp2 (66.6% p-character) hybrid orbitals, fluorine prefers the sp3 hybrid orbital of carbon having relatively smaller s-character.
 Idealised orientation of the electron pairs leading to the minimum repulsion around the central atom
Idealised orientation of the electron pairs leading to the minimum repulsion around the central atom
Effect of lone pair:
(i) Compounds having steric number = 4: The isoelectronic molecules CH4, NH3 and H2O illustrate the effect of lone pairs on molecular shape. Methane has four identical bonds between carbon and each of the hydrogens. When the four pairs of electrons are arranged as far from each other as possible, the result is the familiar tetrahedral shape.
The tetrahedron, with all H–C–H angles measuring 109.5º, has four identical bonds.
Ammonia also has four pairs of electrons around the central atom, three are bonding pairs between N and H and the fourth is a lone pair on the nitrogen.
The nuclei form a trigonal pyramid with the three bonding pairs; with the lone pair, they make a nearly tetrahedral shape. Because each of the three bonding pairs is attracted by two positively charged nuclei (H and N), these pairs are largely confined to the regions between the H and N atoms.
The lone pair, on the other hand, is concentrated near the nitrogen; it has no second nucleus to confine it to a small region of space. Consequently, the lone pair tends to spread out and to occupy more space around the nitrogen than the bonding pairs. As a result, the H–N–H angles are 106.6º, nearly 3º smaller than the angles in methane.
The same principles apply to the water molecule, in which two lone pairs and two bonding pairs repel each other. Again, the electron pairs have a nearly tetrahedral arrangement, with the atoms arranged in a V shape. The angle of largest repulsion between the two lone pairs, is not directly measurable.
However, the lone pair-bonding pair (lp-bp) repulsion is greater than the bonding pair-bonding pair (bp-bp) repulsion, and as a result the H–O–H bond angle is only 104.5º, another 2.1º decrease from the ammonia angles.
The net result is that we can predict approximate molecular shapes by assigning more space to lone electron pairs; being attracted to one nucleus rather than two, the lone pairs are able to spread out and occupy more space.
(ii) Compounds having steric number = 5: For trigonal bipyramidal geometry, there are two possible locations of lone pairs, axial and equatorial. If there is a single lone pair, for example in SF4, the lone pair occupies an equatorial position.
This position provides the lone pair with the most space and minimizes the interactions between the lone pair and bonding pairs. If the lone pair were axial, it would have three 90º interactions with bonding pairs; in an equatorial position, it has only two such interactions, as shown in
Fig. The actual structure is distorted by the lone pair as it spreads out in space and effectively squeezes the rest of the molecule together, giving rise to a seesaw shape.
ClF3 provides a second example of the influence of lone pairs in molecules having a steric number of 5. The two lone pairs are housed in the equatorial hybrid orbitals (where the s-character is maximum) and the more electronegative F atoms go preferably to the axial directions (where there is no s-character).
Of course, the third F atom having no other option will have to occupy the third equatorial positions. The distortion from lone pair repulsion causes the axial fluorine atoms to be bent away from a linear arrangement so that the molecule is a slightly “bent T”.
Effect of Multiple Bonds: The VSEPR model considers double and triple bonds to have slightly greater repulsive effects than single bonds because of the repulsive effect of p electrons. For example, the H3C-C-CH3 angle in (CH3)2C=CH2 is smaller and the H3C-C=CH2 angle is larger than the trigonal 120º.
The d-compounds of steric number 5, SF4, BrF3, and XeF2. Also multiple bonds, like lone pairs, tend to occupy more space than single bonds and to cause distortions that in effect squeeze the reouble bonds to oxygen in SOF4, ClO2F3 and XeO3F2 are all equatorial, as are the lone pairs in the matching st of the molecule together.
Electronegativity and bond angles:
(i) Many bond angles can be explained by either electronegativity or size arguments. Molecules that have a larger difference in electronegativity values between their central and outer atoms have smaller bond angles.
The atom with larger electronegativity draws the electrons towards itself and away from the central atom, reducing the repulsive effect of those bonding electrons. Thereby decreasing bond angle
(ii) The compounds containing fluorine have smaller angles than those containing chlorine, which in turn have smaller angles than those containing bromine or iodine. As a result the lone pair effect is relatively larger and forces smaller bond angles.
The same result is obtained if size is considered; as the size of the outer atom increases in the F < Cl < Br < I series, the angle increases (non bonded electron pair repulsion-bond angle increases with increase in the size of electronegative atom).
(iii) As the central atom becomes more electronegative, it pulls electron in bonding pair more strongly toward itself. This effect increases the concentration of bonding pair electrons near the central atom, causing the bonding pairs to repel each other more strongly, increasing the bond angles. In these situations, the compound with the most electronegative central atom has the largest bond angle.
The size of the central atom can also be used to determine the angles in the series. When the central atom is larger, all the electron pairs are naturally at greater distances from each other. However, the effect is greater for the bonded pairs, which are pulled away from the central atom by outer atoms.
This leads to a relatively larger repulsive effect by the lone pairs and decreasing angles in the order O > S > Se > Te and N > P > As > Sb.
Table: Shape of some molecules having stereochemically active lone pairs according to VSEPR theory
Comparison of bond length (i) More than no. of electronegative element, bond length decreases.
(ii) Always compare bond length of common atoms
Bond length of N–H & N–F are not comparable
Isoelectronic Principle Isoelectronic species usually have the same structure. This may be extended to species with the same number of valence electrons thus NH4+, BH4– & CH4 are tetrahedrals and CO2, N3– & NO2+ are linear.
Limitations of VSEPR Theory
(i) VSEPR theory cannot explain the shapes of molecules which have very polar bonds e.g. Li2O should have the same structure as H2O but in fact it is linear.
(ii) This theory is unable to explain the shapes of molecules having extensive delocalised p-electron systems(iii) This theory cannot explain the shapes of certain molecule which have an inert-pair of electrons.
(iv) This theory is not able to predict the shapes of certain transition metal complexes.
|
50 videos|92 docs|41 tests
|
FAQs on (VSEPR) Theory & Shapes of Molecules - Inorganic Chemistry
| 1. What is VSEPR theory and how does it relate to the shapes of molecules? |  |
| 2. How does the number of electron pairs around a central atom affect the shape of a molecule? |  |
| 3. What is the difference between electron pair geometry and molecular geometry? |  |
| 4. How does the concept of hybridization relate to molecular shapes? |  |
| 5. Are there any exceptions to the VSEPR theory? |  |

















