Absorption Spectra & Emission Spectrum | Chemistry for EmSAT Achieve PDF Download
Atomic spectra involves examining atoms (including atomic ions) by observing how they interact with electromagnetic radiation. Refraction of light is a well-known phenomenon. As light transitions from one medium to another, it bends either towards or away from the normal line. This bending, known as refraction, is primarily due to variations in the speed of light in different mediums. The speed of light is contingent upon the properties of the medium it traverses.
Let's explore the phenomenon of white light dispersion using a prism, and delve into the concepts of emission and absorption spectra.
Emission Spectrum
When electromagnetic radiation interacts with atoms and molecules, the electrons within these entities can absorb energy and transition to higher energy states, disrupting their stability. To restore stability, they must return to lower energy states from the higher ones. To achieve this transition, atoms and molecules emit radiation across different regions of the electromagnetic spectrum. This emitted radiation spectrum, originating from excited electrons within atoms or molecules, is termed as an emission spectrum.

Absorption Spectrum
- Upon observing the passage of a ray of white light through a prism, we note it undergoes refraction twice. Initially, it refracts as it transitions from the less dense medium (air) to the denser medium (glass), and subsequently, as it moves from the denser medium (glass) back to the less dense medium (air).
- Consequently, a band of colors, known as a spectrum, emerges from the white light. Upon closer examination, it becomes apparent that the color with the shortest wavelength deviates the most, and conversely, the color with the longest wavelength experiences the least deviation. Thus, we observe a spectrum spanning from red to violet, with red, having the longest wavelength, undergoing the least deviation.
- This type of spectrum is termed a continuous spectrum, as violet transitions seamlessly into blue, blue into green, and so forth.
However, the emission spectrum of atoms in the gas phase do not exhibit a continuous spread of wavelength from one colour to others. Rather, the emitted light consists of a specific wavelength having dark spaces existing between them. Such kind of spectra is known as atomic spectra or line spectra.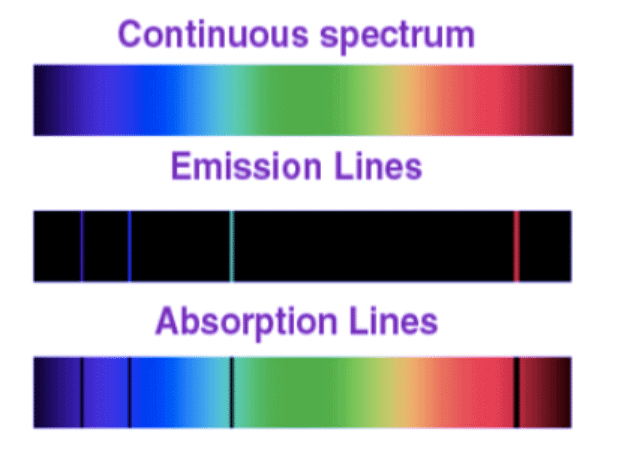
Emission Spectrum & Absorption Spectrum
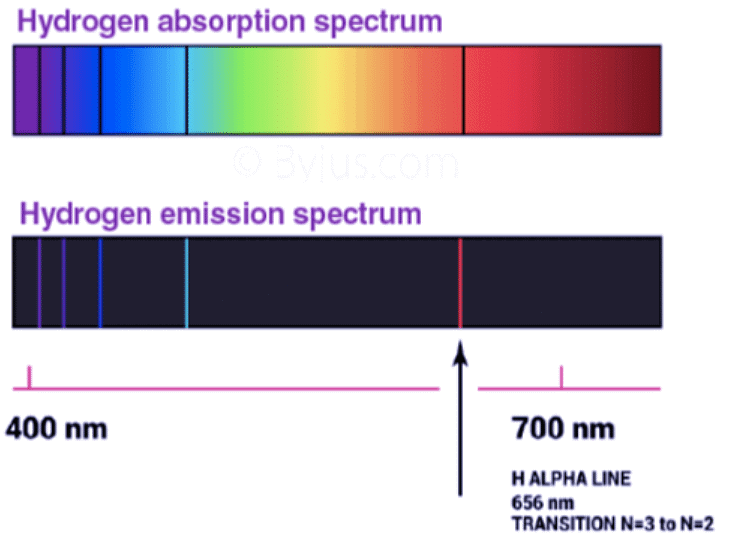
- An absorption spectrum is like a photographic negative of an emission spectrum.
- For observing the absorption spectrum, electromagnetic radiations are bombarded on a sample that absorbs radiation of certain wavelengths.
- The wavelength of radiation absorbed by the matter contributes to the missing wavelength which leaves dark spaces in the bright continuous spectrum.
- Each element has its unique line emission spectrum. The study of the emission spectrum or absorption spectrum is better known as spectroscopy.
|
195 videos|213 docs|157 tests
|
FAQs on Absorption Spectra & Emission Spectrum - Chemistry for EmSAT Achieve
| 1. What is an emission spectrum? |  |
| 2. What is an absorption spectrum? |  |
| 3. How are emission and absorption spectra related? |  |
| 4. How can emission and absorption spectra be used in chemistry? |  |
| 5. Can emission and absorption spectra be used in other scientific fields besides chemistry? |  |

|
Explore Courses for EmSAT Achieve exam
|

|
















